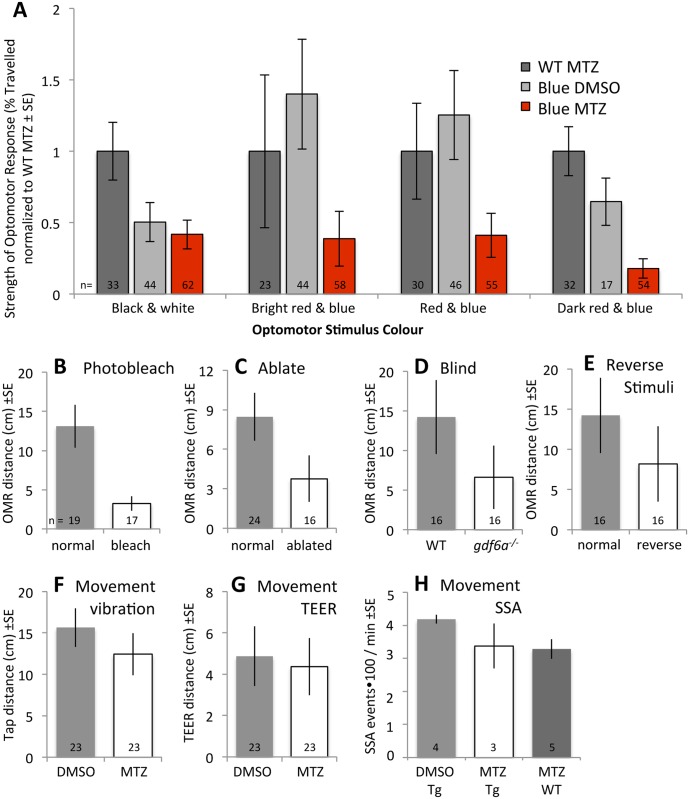
Fig 8. Validation of OMR stimulus as a visually mediated behaviour.
The response of zebrafish larvae to our OMR stimuli comprised of blue and dark red bars was used as a measure of visual function. We validated this conclusion in several ways. A. 24 hours following drug application on transgenic fish (= blue cone ablation, red bars), responses of zebrafish larvae were observed relative to control fish (grey bars) with various stimuli. Stimuli are detailed in S1 Fig, and consisted of typical OMR stimuli of black & white bars, or alternatively blue bars with intervening red bars of various saturation. All stimuli presented were able to distinguish the visually mediated behaviour of larval zebrafish with blue cones ablated relative to controls. The blue and dark red stimuli pair evoked responses that were least variable between individuals and showed the largest magnitude of change to both control treatments, and thus was selected as the stimulus for the majority of our work. B. Larvae acutely blinded by UV light, presumably bleaching their photopigments, responded less than their unblinded siblings (p<0.05). C. Larvae showed photoreceptor degeneration one day following blinding by intense UV light (S2 Fig) and responded less than their unblinded siblings (p<0.05). D. Blind fish have reduced responses to our OMR stimulus. gdf6a-/- mutants, which have previously been shown to exhibit reduced OMR response with typical stimuli, presumably due to overt microphthalmia and cone photoreceptor degeneration, responded less to our OMR stimulus relative to their normophthalmic sighted gdf6a+/- siblings (p<0.05). E. Larvae responding to our OMR stimuli moved through the arena more when stimuli were presented in a typical fashion (described in S1 Fig) compared to when stimuli were presented moving in the opposite direction (p<0.05 by t-test). The latter was predicted to stimulate larvae to remain in their initial position, thus reducing the distance between their initial and final positions, and this prediction was met. F. Alternative stimuli were applied to test for any potential movement deficits induced by combining our transgene and MTZ prodrug. A tapping stimulus delivered to the same computer monitor (that was otherwise used to deliver OMR stimuli) induced equivalent amounts of movement in Tg[sws2:nfsb-mCherry] larvae treated with MTZ (= “Blue MTZ” in later figures) as in their siblings treated with DMSO (p = 0.32). G. As per panel F, but potential movement deficits were assessed by Touch Evoked Escape Response (TEER). Transgenic larvae with blue cones ablated did not move less than larvae treated with vehicle DMSO (p = 0.34). H. Spontaneous Swim Assay records movement of 10 fish per trial for ten minutes (see S3 Fig). Transgenic Tg[sws2:nfsb-mCherry] larvae treated with MTZ did not move less compared to when treated with DMSO or compared to wild type treated with MTZ. Statistical comparisons were made via t-test. Sample sizes (n = number of larvae individually tested, except in panel H sample sizes = number of groups of ten larvae tested) are indicated.