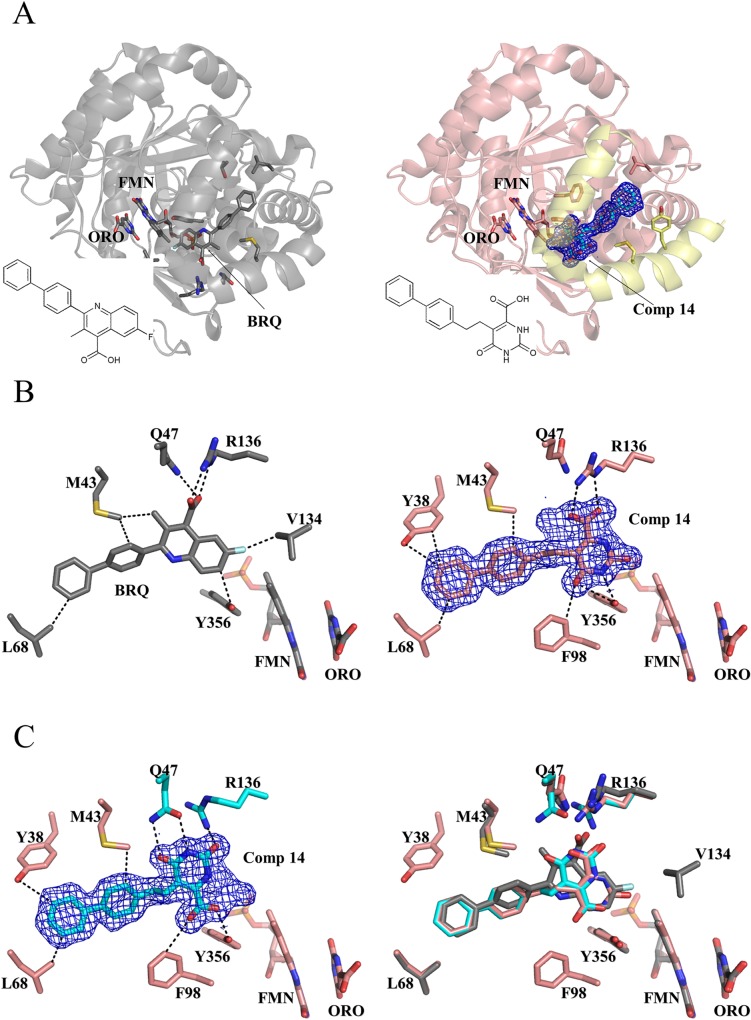
Fig 6. Overlapping binding site between brequinar analog and 14.
(A) Overall structure of HsDHODH complexed with brequinar analog (BRQ, left, gray, 1D3G) [18] and 14 (right, pink, 3W7R). The N-terminal region, which attaches the protein to the mitochondrial inner membrane and proposed to be the ubiquinone reduction site, is highlighted in yellow. (B) Binding modes of the brequinar analog (left, 1D3G)[18] and 14 (right). BRQ, 14, FMN, ORO, and key residues are shown in stick. Interactions within 3.5 Å are shown as dashed lines. Note the interaction of the carboxylate group from 14 with R136 (right), as in the BRQ analog (left), resulting in a “brequinar-like” binding mode. (C) The carboxylate group of 14 interacts with Y356 in a “non-brequinar-like” binding mode (left). BRQ and the dual binding modes of 14 were superposed in the right figure. Color codes for nitrogen, oxygen, sulfur, and fluorine are blue, red, dark yellow, and white, respectively. Carbon atoms from BRQ analog and 14 bound in a brequinar-like mode are gray and salmon, respectively. Carbon atoms from 14 and the two residues (Q47 and R136) that change their conformation in a non-brequinar-like binding mode are colored in cyan. The electron density map of 14 is shown as blue mesh and contoured at a 1 σ level.