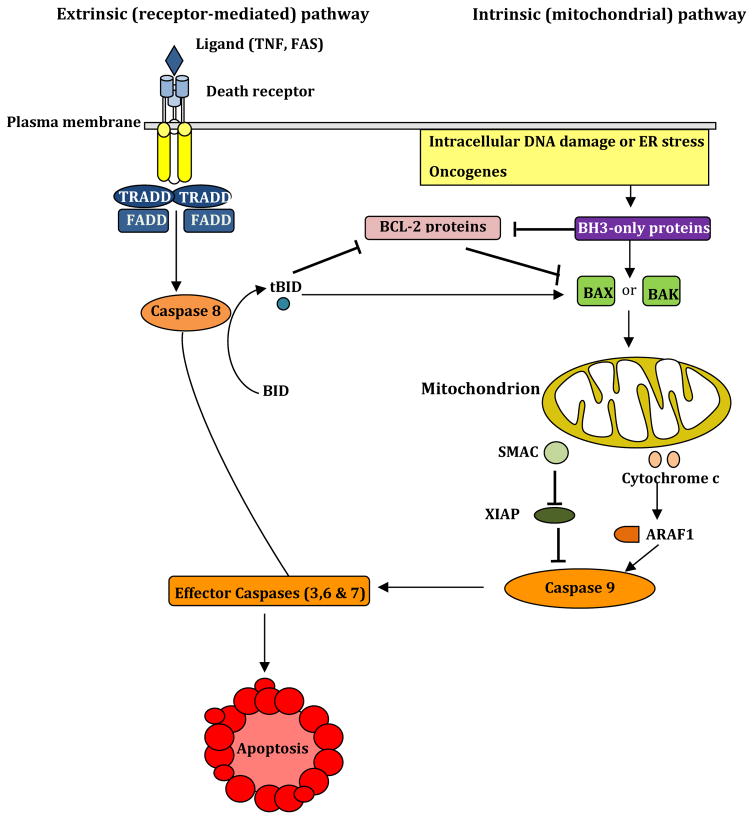
Figure 3. The extrinsic (receptor-mediated) and intrinsic (mitochondrial) pathways of apoptosis.
The receptor-mediated (extrinsic) pathway of apoptosis is initiated when ligands of TNF-α family such as FAS and TNF-α bind their plasma membrane-bound death receptors, this binding leads to activation of caspase-8 via TNFR-associated death domain (TRADD) and FAS-associated death domain (FADD) proteins. TRADD is required for induction of apoptosis in response to TNF-α a binding to TNFR. TRADD and FADD activate effector caspases (caspase-3, 6 and 7) causing apoptosis. Moreover, caspase-8 generates the active truncated form of BID (tBID) that inhibits pro-survival BCL-2-like proteins (BCL-2) and engages in the mitochondrial pathway. However, this engagement is only apparent in certain cells such as liver cells (type 2 cells), while it is absent in type 1 cells, such as thymocytes. Several stimuli such as DNA damaging agents, oncogenes, and ER stress stimulate the mitochondrial (intrinsic) pathway via activation of BH3-only family of proteins that inhibit pro-survival BCL-2 like proteins. Such inhibition leads to activation of pro-apoptotic BAX and BAK and subsequent disruption of the mitochondrial membrane and the release of cytochrome c and SMAC (second mitochondria-derived activator of caspases). Cytochrome c activates caspase-9 via APAF1 (apoptotic protease-activating factor 1) that activates effector caspases, whereas SMAC blocks the caspase inhibitor protein XIAP (X-linked inhibitor of apoptosis protein).