Fig. 2. Gram-positive heme uptake systems.
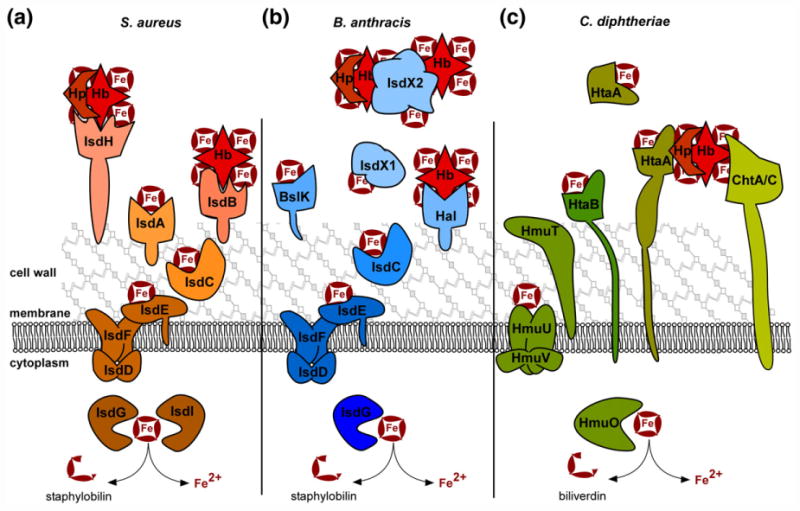
The iron-regulated surface determinant (Isd) systems for heme acquisition in S. aureus and B. anthracis, as well as the non-Isd systems of C. diphtheriae are diagrammed. Host Hb, Hp-bound Hb, and free heme (Fe-containing ring) can serve as heme sources during infection. (a) In S. aureus, IsdH is the primary Hp–Hb receptor and IsdB is the principle Hb receptor. Both are sortase-linked on the surface of the cell wall, bind host hemoproteins with NEAT domains, and extract heme using additional NEAT domains. IsdA can bind free heme or accept heme from IsdB and IsdH. Heme is transferred to IsdC, which is embedded in the cell wall and transits heme to the membrane complex IsdDEF. IsdDEF transports heme to the cytoplasm for utilization intact or for degradation by the heme oxygenases IsdG/I. (b) Similarly, B. anthracis uses Isd proteins to acquire heme. IsdX1 and IsdX2 are secreted hemophores that bind Hb, Hp–Hb, or free heme as depicted. IsdX2, which has five NEAT domains, may also serve as a heme storage protein. Additionally, the sortase anchored Hal serves as a Hb receptor on the cell surface and uses its NEAT and leucine-rich repeat domains to acquire heme. BslK is cell wall associated and binds heme via its NEAT domain. IsdC transports heme to the IsdDEF membrane importer for utilization or degradation by IsdG. (c) C. diphtheriae utilizes a unique set of heme uptake proteins for heme utilization. HtaA is a cell wall spanning lipoprotein that can acquire heme from Hp–Hb in conjunction with ChtA or ChtC. HtaB can bind free heme or accept heme transfer from HtaA and transfers heme to the HmuTUV membrane transporter. A portion of HtaA may also serve as a secreted hemophore. C. diphtheriae HmuO heme oxygenase can liberate iron from imported heme.
