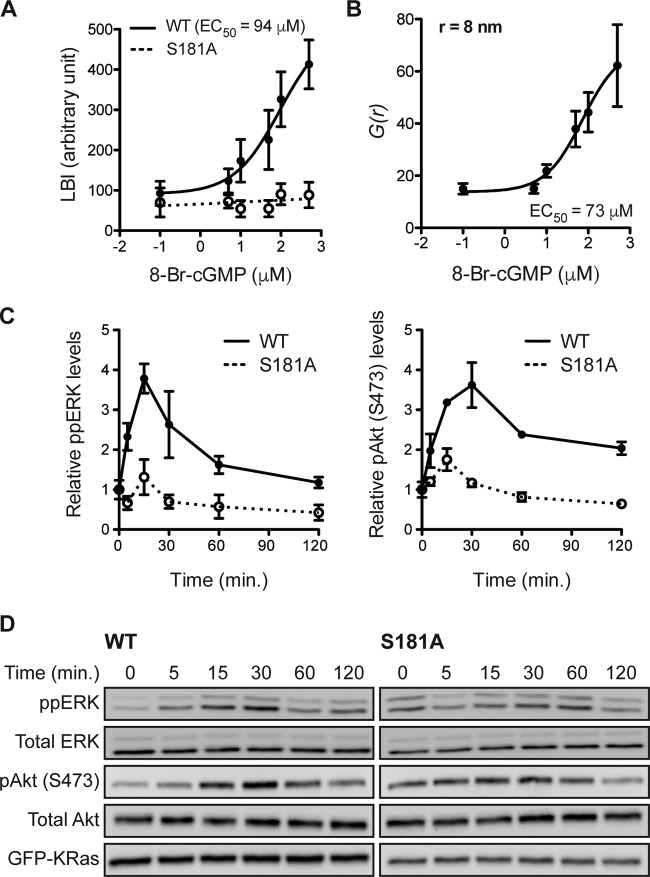
FIG 6.
K-Ras phosphorylation differentially regulates PM interactions. (A) Intact PM sheets prepared from MDCK cells coexpressing mGFP–K-RasG12V S181D and mCherry–K-RasG12V (solid line) or mRFP–K-RasG12V S181A (dotted line) after 8-Br-cGMP treatment for 6 h were labeled with anti-GFP–6-nm gold and anti-RFP–2-nm gold, respectively. The extent of coclustering of the two proteins was analyzed using bivariate K-functions, shown as the summary statistic LBI. The graph shows the mean LBI values ± SEM from ≥20 sheets for each data point. The LBI value for coclustering of mGFP–K-RasG12V with mCherry–K-RasG12V was measured to be 355, which implies that 500 μM 8-Br-cGMP induces the nearly complete overlap of the mGFP–K-RasG12V S181D and mCherry–K-RasG12V gold populations, consistent with ∼100% mCherry–K-RasG12V phosphorylation. (B) Intact PM sheets from MDCK cells expressing mGFP–K-RasG12V were treated for 6 h with 8-Br-cGMP, labeled with anti-GFP–4.5-nm gold, and visualized by EM. Spatial organization was analyzed at each 8-Br-cGMP concentration using the pair correlation function g(r) (r = 8 nm; data are means ± SEMs for 20 PM sheets). (C and D) MDCK cells stably expressing mGFP–K-RasG12V (wild type [WT]) or mGFP–K-RasG12V S181A (S181A) were serum starved overnight and treated with 100 μM 8-Br-cGMP for various time intervals in the absence of serum. Cell lysates were quantitatively immunoblotted for ppERK or pAkt (S473). The graphs shows the means ± SEMs from three independent experiments (C), with representative blots being shown (D). Total ERK, total Akt, and GFP blots were included as loading controls.