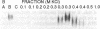Abstract
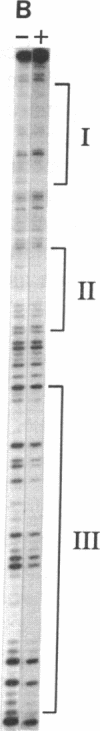
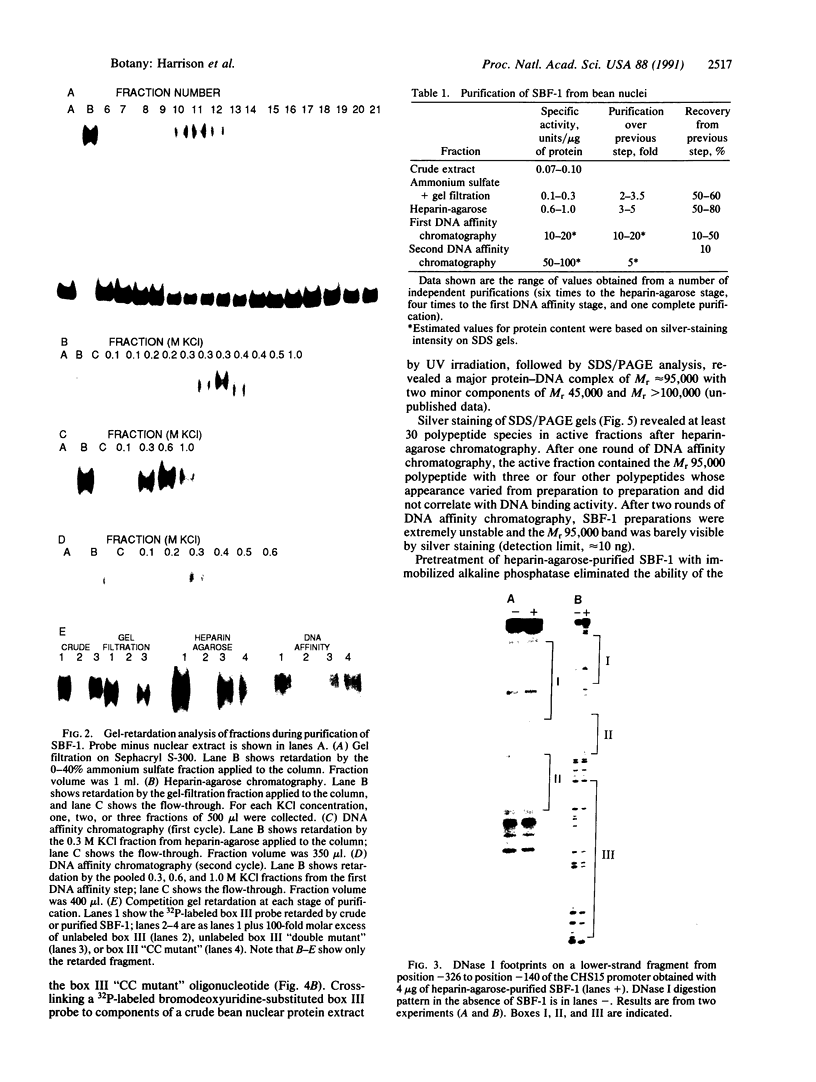
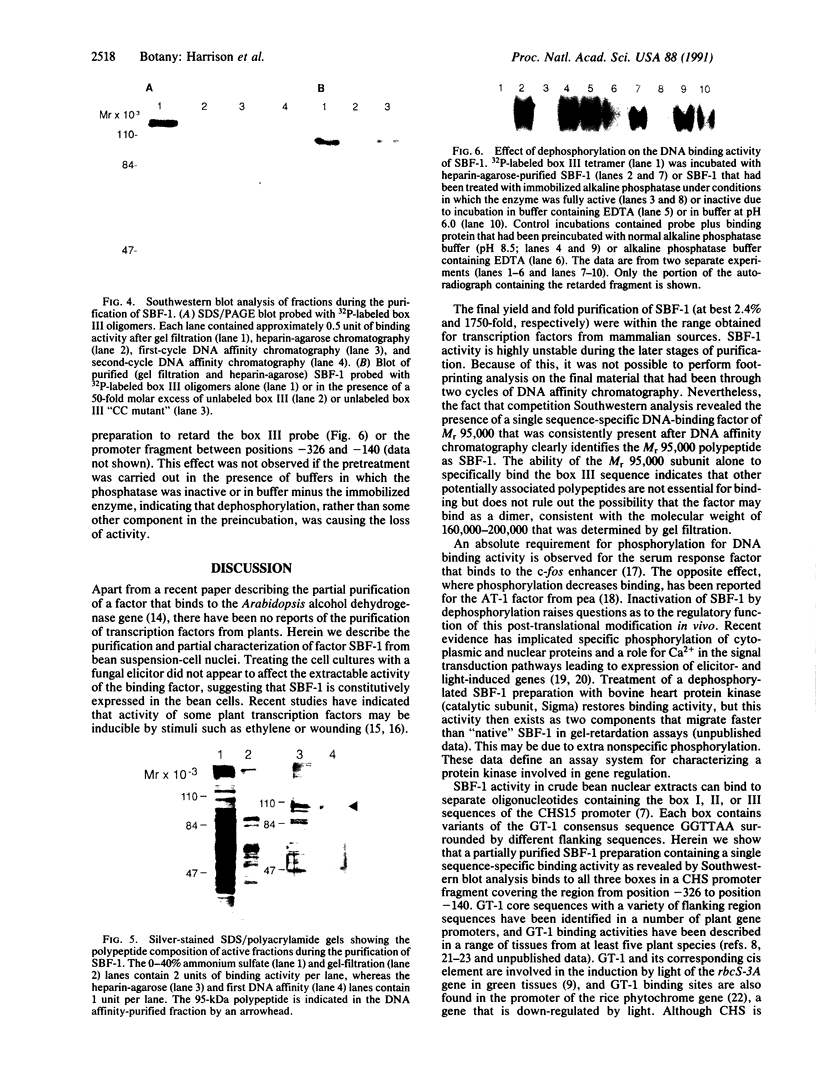
The chalcone synthase (EC 2.3.1.74) gene promoter from the bean Phaseolus vulgaris L. contains a silencer element between positions -140 and -326 fro the transcription start site that is functional in electroporated soybean protoplasts. This element contains three binding sites for a bean nuclear factor (SBF-1) with DNA sequence recognition properties that are very similar to those of nuclear factor GT-1. By using a synthetic tetramer of one of the binding sites as probe, we have purified sequence-specific SBF-1 activity approximately 1750-fold from suspension-cell nuclei, by using a combination of ammonium sulfate precipitation, gel filtration, heparin-agarose chromatography, and sequence-specific DNA affinity chromatography. The factor exhibited an apparent molecular weight of 160,000-200,000 on the basis of gel filtration. A subunit molecular weight of approximately 95,000 was determined from SDS/polyacrylamide gel electrophoretic analysis of purified fractions, followed by Southwestern blot analysis (a protein blot probed with oligonucleotide probes), and from UV-cross-linking experiments. The factor lost DNA-binding activity on treatment with alkaline phosphatase. We discuss the properties of SBF-1 in relation to the functionality of GT-1 binding sequences in plant genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benfey P. N., Ren L., Chua N. H. Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J. 1990 Jun;9(6):1677–1684. doi: 10.1002/j.1460-2075.1990.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Cashmore A. R. Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation. Plant Cell. 1989 Nov;1(11):1069–1077. doi: 10.1105/tpc.1.11.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle A. J., Ferl R. J. Characterization of the Arabidopsis Adh G-box binding factor. Plant Cell. 1990 Jun;2(6):547–557. doi: 10.1105/tpc.2.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Harrison M. J. Activation, structure, and organization of genes involved in microbial defense in plants. Adv Genet. 1990;28:165–234. doi: 10.1016/s0065-2660(08)60527-1. [DOI] [PubMed] [Google Scholar]
- Dron M., Clouse S. D., Dixon R. A., Lawton M. A., Lamb C. J. Glutathione and fungal elicitor regulation of a plant defense gene promoter in electroporated protoplasts. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6738–6742. doi: 10.1073/pnas.85.18.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin P. M., Sarokin L., Memelink J., Chua N. H. Molecular light switches for plant genes. Plant Cell. 1990 May;2(5):369–378. doi: 10.1105/tpc.2.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Kay S. A., Chua N. H. Sequence-specific interactions of a pea nuclear factor with light-responsive elements upstream of the rbcS-3A gene. EMBO J. 1987 Sep;6(9):2543–2549. doi: 10.1002/j.1460-2075.1987.tb02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S. A., Keith B., Shinozaki K., Chye M. L., Chua N. H. The rice phytochrome gene: structure, autoregulated expression, and binding of GT-1 to a conserved site in the 5' upstream region. Plant Cell. 1989 Mar;1(3):351–360. doi: 10.1105/tpc.1.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam E., Benedyk M., Chua N. H. Characterization of phytochrome-regulated gene expression in a photoautotrophic cell suspension: possible role for calmodulin. Mol Cell Biol. 1989 Nov;9(11):4819–4823. doi: 10.1128/mcb.9.11.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Chua N. H. GT-1 binding site confers light responsive expression in transgenic tobacco. Science. 1990 Apr 27;248(4954):471–474. doi: 10.1126/science.2330508. [DOI] [PubMed] [Google Scholar]
- Miskimins W. K., Roberts M. P., McClelland A., Ruddle F. H. Use of a protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferrin receptor gene. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6741–6744. doi: 10.1073/pnas.82.20.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm C. J., Costa M. A., An G., Ryan C. A. Wound-inducible nuclear protein binds DNA fragments that regulate a proteinase inhibitor II gene from potato. Proc Natl Acad Sci U S A. 1990 Jan;87(2):603–607. doi: 10.1073/pnas.87.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Dutta A., Cromlish J. A., Roeder R. G. Phosphorylation of serum response factor, a factor that binds to the serum response element of the c-FOS enhancer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7206–7210. doi: 10.1073/pnas.85.19.7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder T. B., Hedrick S. A., Bell J. N., Liang X. W., Clouse S. D., Lamb C. J. Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet. 1987 Dec;210(2):219–233. doi: 10.1007/BF00325687. [DOI] [PubMed] [Google Scholar]
- Schmid J., Doerner P. W., Clouse S. D., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a bean chalcone synthase promoter in transgenic tobacco. Plant Cell. 1990 Jul;2(7):619–631. doi: 10.1105/tpc.2.7.619. [DOI] [PMC free article] [PubMed] [Google Scholar]