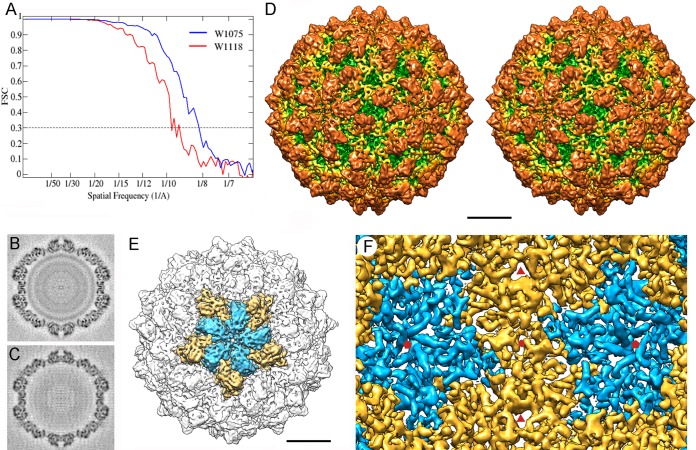
FIG 2.
Three-dimensional cryo-EM of RnQV1 virions. (A) Assessment of the resolution of full (W1075) and empty (W1118) RnQV1 reconstructions. FSC resolution curves were calculated for full (blue) and empty (red) capsids. Each set of particle images was subdivided randomly into two subsets, and independent reconstructions were computed from the data. Resolutions for which correlations were <0.3 are indicated. For the 0.3 threshold, the values for full and empty RnQV1 capsids were 8.2 and 9.1 Å, respectively. (B and C) Central sections from the 3D reconstruction of full (B) and empty (C) capsids, viewed along a 2-fold axis. Protein and RNA are dark. The two protein shells are virtually identical, and the RNA density of the full capsid is seen as concentric circles inside the capsid. (D) Stereo view of the radially color-coded outer surface of the full capsid, viewed along a 2-fold axis of icosahedral symmetry. The most prominent features are 120 outward-protruding densities (orange). The map is contoured at 2.5 σ above the mean density. Bar = 100 Å. (E) Surface-shaded virion capsid viewed along an icosahedral 5-fold axis showing the five A (blue) and B (yellow) structural subunits in a pentamer. (F) Inner surface of the RnQV1-W1075 capsid (for clarity, only the density between 145- and 210-Å radii is shown). Icosahedral-symmetry axes are indicated (red symbols).