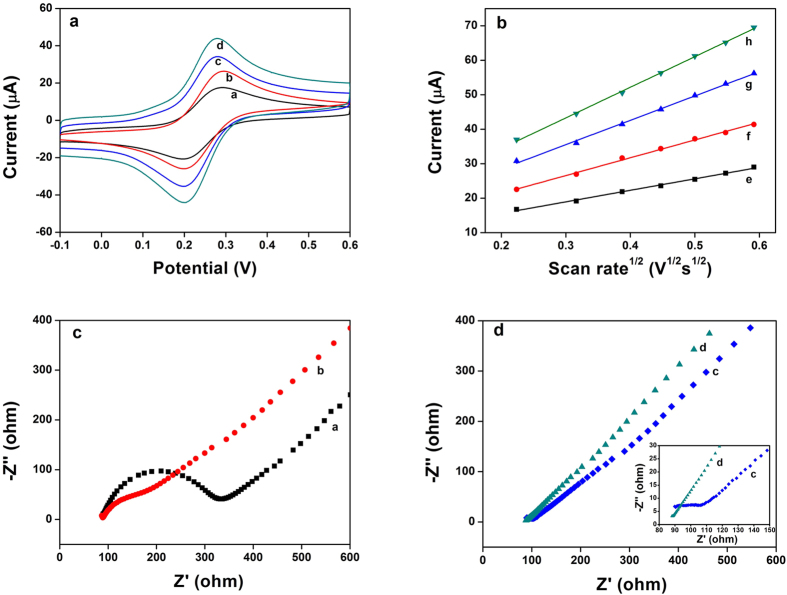
Figure 4.
(a) Cyclic voltammograms of GCE (curve a), MWCNTs/GCE (curve b), MWCNTs-Fe3O4/GCE (curve c) and TOAB/MWCNTs-Fe3O4/GCE (curve d) in 0.1 M KCl solution containing 1 mM [Fe(CN)6]3−/4− at a scan rate of 100mVs−1. (b) The plot of anodic peak current of [Fe(CN)6]3−/4− (1 mM) vs. square root of scan rate for: GCE(curve e), MWCNTs/GCE (curve f), MWCNTs-Fe3O4/GCE (curve g)and TOAB/MWCNTs-Fe3O4/GCE (curve h). (c,d) Electrochemical impedance spectroscopy of GCE (curve a), MWCNTs/GCE (curve b), MWCNTs-Fe3O4/GCE (curve c) and TOAB/MWCNTs-Fe3O4/GCE (curve d) in 0.10 M KCl containing 5 mM [Fe(CN)6]3−/4−, frequency range: 0.1 kHz to 10 kHz with amplitude of 5 mV. Inset: Electrochemical impedance spectroscopy of MWCNTs-Fe3O4/GCE (curve c) and TOAB/MWCNTs-Fe3O4/GCE (curve d) at the high-frequency region.