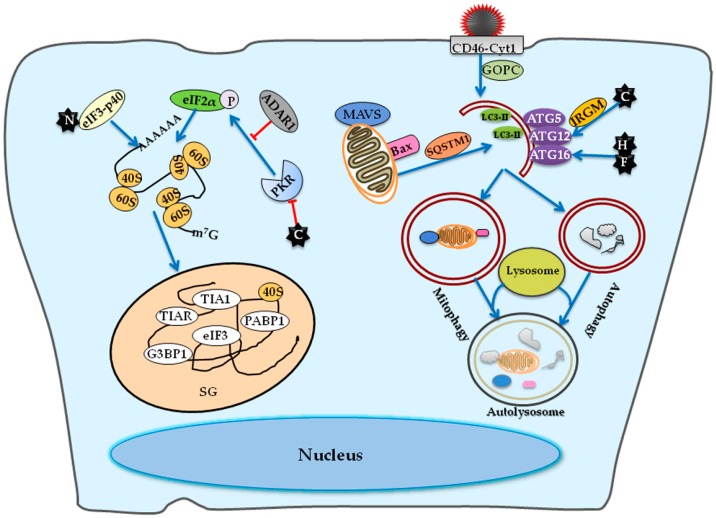
Figure 3.
A diagram of host factors involved in MeV inducible stress granule formation and autophagy. The C protein suppresses the activation of PKR, so a mutant MeV lacking C protein expression rather than the wild-type MeV induces the stress granule (SG) formation. The N protein binds to the eIF3-p40 and then promotes SG formation. As a suppresser of PKR, ADAR1 suppresses the PKR-dependent SG formation. The infection of Edmonston-MeV strain initiates an early but transient autophagy wave via the engagement of CD46-Cyt-1 in a Golgi-associated PDZ and coiled-coil motif-containing protein (GOPC)-dependent pathway. Moreover, the expression of C protein can induce an autophagic signaling in an immunity-related GTPase M (IRGM)-dependent manner. The expression of H and F proteins in cells expressing one of the cellular receptors is also sufficient to induce autophagy. The Edmonston-MeV strain also exploits the selective mitophagy via recognition of damaged mitochondria by autophagic receptor SQSTM1, resulting in the degradation of mitochondrion-tethered mitochondrial antiviral-signaling protein (MAVS) and subsequent attenuation of RIG-I/MDA5-mediated responses. The arrows indicate the activation of innate immune responses while the T-ended arrows indicate the repression by MeV.