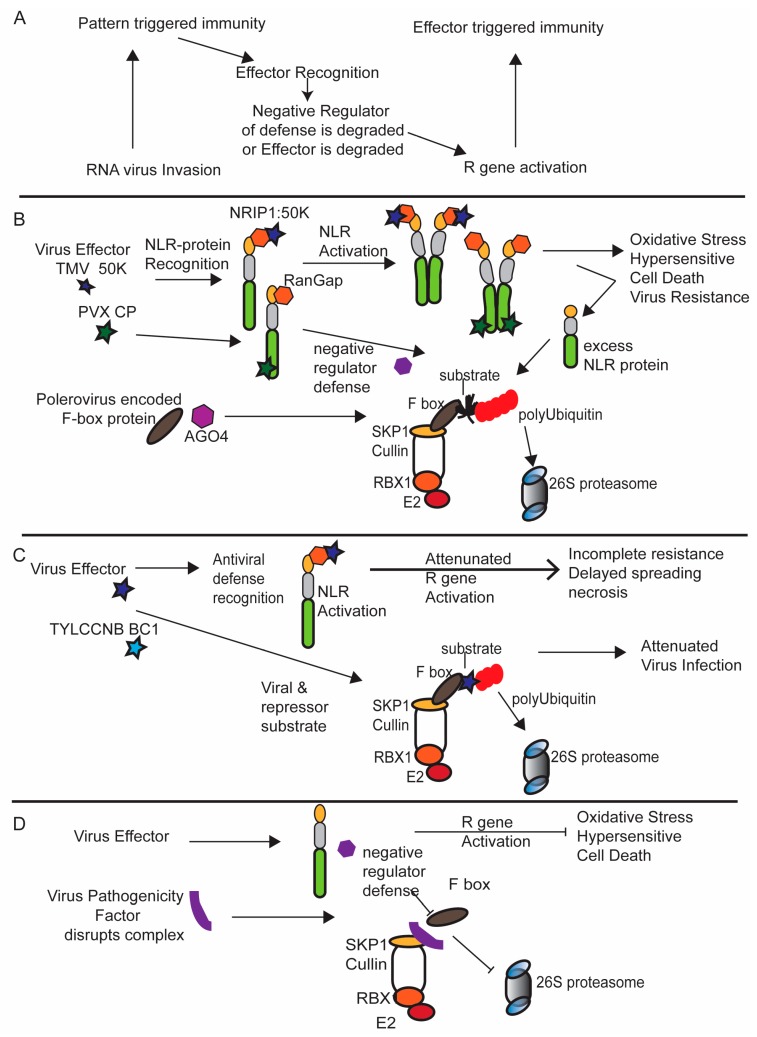
Figure 2.
Diagrammatic representation of viral protein interactions with the UPS with respect to the molecular arms race; (A) zig-zag model demonstrating the co-evolution of viruses with pathogen associated molecular patterns (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI); (B) two examples of tobacco mosaic virus (TMV) and potato virus X (PVX) effectors that activate N-protein or Rx-protein (NLR proteins) leading to activation of ETI and a hypersensitive cell death. With respect to N-protein mediated resistance, nuclear receptor interaction protein (NRIP) is a co-factor that binds the viral 50K effector. The co-factor for Rx is Ran–Gap. Activation of the respective NLR proteins involves effector recognition, followed by dimerization or oligomerization. This is followed by oxidative stress, hypersensitive response and virus resistance. This model suggests that unknown factors that may serve as a negative regulator of the NLR protein, which blocks autoactivation, may be relayed to the SCF complex for ubiquitination and 26S proteasome degradation. In which case, the SCF complex is central to regulating NLR protein activation. Another method for protecting the cell from auto-activation involves routine turnover of NLR proteins that may be excessive or have completed the necessary activation of defense responses. With regard to PTI, poleroviruses encode an F-box protein that binds to Argonaute 4 (AGO4), relaying it to the SCF complex for ubiquitination and degradation. This serves to compromise PTI; (C) a proposed model for degradation of viral effectors by the SCF complex or ERAD machinery to evade immune recognition; and (D) certain viruses are known to interact with S-Phase Kinase-Associated Protein 1 (SKP1) but do not appear to function as F-box proteins. One possibility is that these proteins insert into the SCF complex and disrupt its ability to function in the degradation of negative regulators of defense as in panel A. In this scenario, the immune system may be blocked.