Abstract
This study was designed to establish if Curcumin (CM) alleviates Aflatoxin B1 (AFB1)-induced hepatotoxic effects and to determine whether alteration of the expression of cytochrome P450 (CYP450) isozymes is involved in the regulation of these effects in chick liver. One-day-old male broilers (n = 120) were divided into four groups and used in a two by two factorial trial in which the main factors included supplementing AFB1 (< 5 vs. 100 μg/kg) and CM (0 vs. 150 mg/kg) in a corn/soybean-based diet. Administration of AFB1 induced liver injury, significantly decreasing albumin and total protein concentrations and increasing alanine aminotransferase and aspartate aminotransferase activities in serum, and induced hepatic histological lesions at week 2. AFB1 also significantly decreased hepatic glutathione peroxidase, catalase, and glutathione levels, while increasing malondialdehyde, 8-hydroxydeoxyguanosine, and exo-AFB1-8,9-epoxide (AFBO)-DNA concentrations. In addition, the mRNA and/or activity of enzymes responsible for the bioactivation of AFB1 into AFBO—including CYP1A1, CYP1A2, CYP2A6, and CYP3A4—were significantly induced in liver microsomes after 2-week exposure to AFB1. These alterations induced by AFB1 were prevented by CM supplementation. Conclusively, dietary CM protected chicks from AFB1-induced liver injury, potentially through the synergistic actions of increased antioxidant capacities and inhibition of the pivotal CYP450 isozyme-mediated activation of AFB1 to toxic AFBO.
Keywords: curcumin, aflatoxin B1, CYP450, AFBO–DNA, chicks
1. Introduction
Aflatoxins (AF) are secondary fungal metabolites that are largely produced by the fungi Aspergillus flavus and Aspergillus parasiticus [1,2]. Among the various dangerous AF and their metabolites, aflatoxin B1 (AFB1) is the most toxic, exhibiting harmful hepatotoxic, teratogenic, mutagenic, and carcinogenic effects on humans and many species of livestock [3,4,5,6]. It is also classified as a Group I carcinogen [7]. Human or animal consumption of the food or feed contaminated by AFB1 can pose serious problems to their health and productivity, and thus result in significant economic losses [8,9]. The toxic effects of AFB1 are associated with its toxification and detoxification biotransformation pathways. Upon being delivered to the liver, AFB1 is bioactivated by cytochrome P450 (CYP450)—a member of the phase I metabolizing enzymes—into the highly reactive exo-AFB1-8,9-epoxide (AFBO) [3,10]. AFBO can form adducts with DNA and other critical macromolecules, causing toxicity, mutations, and cancer [10]. Meanwhile, AFB1 can induce the generation of reactive oxygen species (ROS), which can lead to oxidative stress, potentially mediated via CYP450 activity [11,12]. On the other hand, AFBO can be detoxified via conjugation with glutathione (GSH) to form a non-toxic adduct, which can be catalyzed by glutathione-S transferases (GSTs), the phase II detoxification enzymes [10].
Curcumin (CM) is a natural polyphenolic compound extracted from rhizomes of Curcuma longa Linn (turmeric), widely used as household spice, natural food colorant, and herbal medicine in many Asian countries for thousands of years [13]. It possesses antioxidant, anti-inflammatory, radio-protective, chemotherapeutic, anti-cancer, and detoxification abilities in laboratory animals and humans [14,15,16,17]. Previous publications have described that CM can effectively mitigate AFB1-induced adverse effects in several animal species [6,15,18,19,20]. Moreover, the protective action of CM against AFB1-induecd adverse effects was further demonstrated by improving the antioxidant capacity [18,19,21,22]. Notably, as different CYP450 isozymes catalyze AFB1 to various metabolites, including the highly toxic AFBO and the less- or non-toxic aflatoxicol, AFM1, AFP1, and AFQ1, examination of the regulation of the proportions of CYP450 isozymes by CM would be valuable [10,23]. Previous studies have described that CM can alter various CYP450 isozymes in vivo and in vitro [24,25,26,27]. Moreover, CM inhibition of AFB1 toxicity has been reported by modulating CYP450 function [14], while the knowledge of which crucial CYP450 isozymes are involved in this process remains unknown. Chicken orthologs of human CYP1A, 2A, and 3A families are the main CYP450 enzymes responsible for the bioactivation of AFB1 into the highly toxic AFBO in chicken [23]. However, there is limited information on the effect of CM on these pivotal CYP450 isozymes that are involved in AFB1 metabolism. Therefore, we selected chickens to investigate whether dietary supplementation of CM mitigated AFB1-induced hepatotoxic effects though the regulation of these key CYP450 isozymes.
2. Results
2.1. Growth Performance, Serum Biochemistry, and Liver Histology
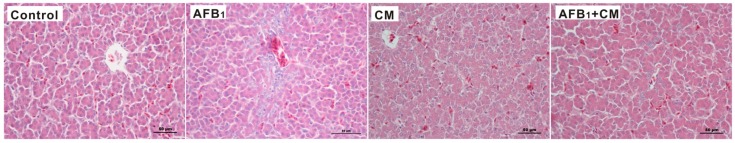
Non-significant differences in average daily feed intake, average daily gain, and feed/gain ratio were observed among the four groups throughout the experiment (Table S1). Serum biochemical parameters were significantly affected by either supplementation of AFB1 or CM at week 2 (Table 1). Compared to the control, the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) increased (p < 0.05) by 33.3% and 43.8% respectively, while the concentrations of albumin (ALB) and total protein (TP) decreased (p < 0.05) by 33.8% and 26.0%, respectively, in the serum of chicks by AFB1 supplementation. Notably, the serum biochemical parameter changes observed in the AFB1 group were prevented in the AFB1 + CM group. However, no significant differences in these serum biochemical parameters were observed among the four groups at week 4 (Table 1). Since the serological results indicated AFB1 only induced liver injury at week 2, we selected samples from week 2 to explore the mechanism. Furthermore, dietary AFB1 exposure induced liver injury as shown through bile duct hyperplasia and necrosis at week 2. Strikingly, the AFB1 + CM group prevented the hepatic injury observed in the AFB1 group (Figure 1).
Table 1.
Effects of dietary AFB1 and CM concentrations on serum biochemical parameters in chicks 1.
| Item | Control | AFB1 | CM | AFB1 + CM |
|---|---|---|---|---|
| Week 2 | ||||
| ALT, U/L | 1.2 ± 0.1 a | 1.6 ± 0.4 b | 1.2 ± 0.3 a,b | 1.3 ± 0.2 a,b |
| AST, U/L | 176.7 ± 27.0 a | 254.2 ± 53.9 b | 178.4 ± 38.1 a | 193.5 ± 39.4 a,b |
| TP, g/L | 17.7 ± 1.1 b | 13.1 ± 2.3 a | 17.3 ± 1.8 b | 19.1 ± 2.0 b |
| ALB, g/L | 7.4 ± 0.3 b | 4.9 ± 1.0 a | 7.1 ± 1.2 b | 8.1 ± 1.0 b |
| Week 4 | ||||
| ALT, U/L | 1.0 ± 0.1 | 1.2 ± 0.3 | 1.4 ± 0.4 | 1.6 ± 0.5 |
| AST, U/L | 217.4 ± 26.8 | 211.2 ± 22.0 | 223.6 ± 37.5 | 245.9 ± 83.4 |
| TP, g/L | 22.8 ± 3.9 | 23.3 ± 2.7 | 21.8 ± 4.4 | 26.3 ± 7.8 |
| ALB, g/L | 9.9 ± 2.1 | 9.8 ± 1.9 | 9.3 ± 2.4 | 11.6 ± 3.7 |
1 Values are expressed as means ± SD (n = 5), and means with different superscript letters differ (p < 0.05). AFB1, aflatoxin B1; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CM, curcumin; TP, total protein. Experimental details of Control and AFB1 groups are given in Sun et al. (2016) [12].
Figure 1.
Photomicrographs of hepatic sections stained with hematoxylin and eosin (400× magnification) of chicks from different treatment groups at week 2. AFB1, aflatoxin B1; CM, curcumin. Experimental details of Control and AFB1 groups are given in Sun et al. (2016) [12].
2.2. Hepatic Antioxidant Parameters and CYP450 Isozyme Activities
After 2 weeks of experimental treatments, the antioxidant parameters and CYP450 isozyme activities were significantly altered by either supplementation of AFB1 or CM (Table 2 and Table 3). Compared to the control, supplementation of dietary AFB1 led to a decrease (0.05) in the activities of glutathione peroxidase (GPX, 13.1%), catalase (CAT, 16.2%), and GSH concentration (30.9%), along with increase (p < 0.05) in concentrations of malondialdehyde (MDA, 100.0%) and 8-hydroxydeoxyguanosine (8-OHdG, 17.9%) in the liver of chicks at week 2, respectively. Interestingly, the antioxidant parameter changes observed in the AFB1 group were prevented in the AFB1 + CM group (Table 2). In addition, supplementation of CM alone increased (p < 0.05) activity of GPX (25%), while it did not affect the other antioxidant parameters, when compared with the control. Meanwhile, the dietary AFB1 supplementation led to an increase (p < 0.05) in the activity of CYP1A1 (270.6%), CYP1A2 (99.4%), CYP2A6 (184.5%), and CYP3A4 (29.2%) in the liver microsomes of chickens, respectively (Table 3). Strikingly, the increased CYP450 isozyme activities observed in the AFB1 group were reduced in the AFB1 + CM group.
Table 2.
Effects of dietary AFB1 and CM concentrations on hepatic antioxidant parameters in chicks at week 2 1.
| Item | Control | AFB1 | CM | AFB1 + CM |
|---|---|---|---|---|
| GPX, U/mg | 127.8 ± 5.1 b | 111.1 ± 10.3 a | 159.9 ± 15.1 c | 159.7 ± 8.9 c |
| SOD, U/mg | 156.8 ± 5.2 a,b | 149.9 ± 9.7 a | 170.2 ± 6.7 b | 162.5 ± 8.7 a,b |
| CAT, U/mg | 13.6 ± 0.9 b | 11.4 ± 1.6 a | 15.4 ± 1.2 b,c | 16.7 ± 1.0 c |
| GST, U/mg | 61.5 ± 1.1 | 60.6 ± 1.9 | 62.3 ± 5.1 | 62.0 ± 2.4 |
| GSH, μmol/g | 48.5 ± 10.1 b | 33.5 ± 3.9 a | 62.8 ± 17.6 b | 53.4 ± 15.6 b |
| MDA, μmol/g | 3.2 ± 0.4 a | 6.4 ± 1.3 b | 2.9 ± 0.5 a | 3.2 ± 0.6 a |
| 8-OHdG, nmol/mg | 152.2 ± 8.1 a | 179.5 ± 5.4 b | 157.9 ± 2.9 a | 156.8 ± 4.7 a |
1 Values are expressed as means ± SD (n = 5), and means with different superscript letters differ (p < 0.05). AFB1, aflatoxin B1; CAT, catalase; CM, curcumin; GPX, glutathione peroxidase; GSH, glutathione; GST, glutathione-S transferases; MDA, malondialdehyde; SOD, superoxide dismutase; 8-OHdG, 8-hydroxydeoxyguanosine. Experimental details of Control and AFB1 groups are given in Sun et al. (2016) [12].
Table 3.
Effects of dietary AFB1 and CM concentrations on the activities of chicken cytochrome P450 (CYP450) orthologs in the liver at week 2 1.
| Item | Control | AFB1 | CM | AFB1 + CM |
|---|---|---|---|---|
| CYP1A1, nmol/(mgprotein·min) | 0.34 ± 0.13 a | 1.26 ± 0.17 c | 0.83 ± 0.15 b | 0.79 ± 0.16 b |
| CYP1A2, nmol/(mgprotein·min) | 1.71 ± 0.45 a | 3.41 ± 0.49 b | 2.45 ± 0.63 a,b | 1.95 ± 0.55 a |
| CYP2A6, nmol/(mgprotein·min) | 2.07 ± 0.30 a | 5.89 ± 1.37 b | 3.00 ± 1.18 a | 3.08 ± 0.63 a |
| CYP3A4, nmol/(mgprotein·min) | 26.93 ± 2.22 a | 34.79 ± 3.06 b | 24.86 ± 1.51 a | 22.60 ± 2.26 a |
1 Values are expressed as means ± SD (n = 5), and means with different superscript letters differ (p < 0.05). AFB1, aflatoxin B1; CM, curcumin; CYP1A1, Cytochrome P450 1A1; CYP1A2, Cytochrome P450 1A2; CYP2A6, Cytochrome P450 2A6; CYP3A4, Cytochrome P450 3A4. Experimental details of Control and AFB1 groups are given in Sun et al. (2016) [12].
2.3. Hepatic AFBO–DNA Adduct Contents
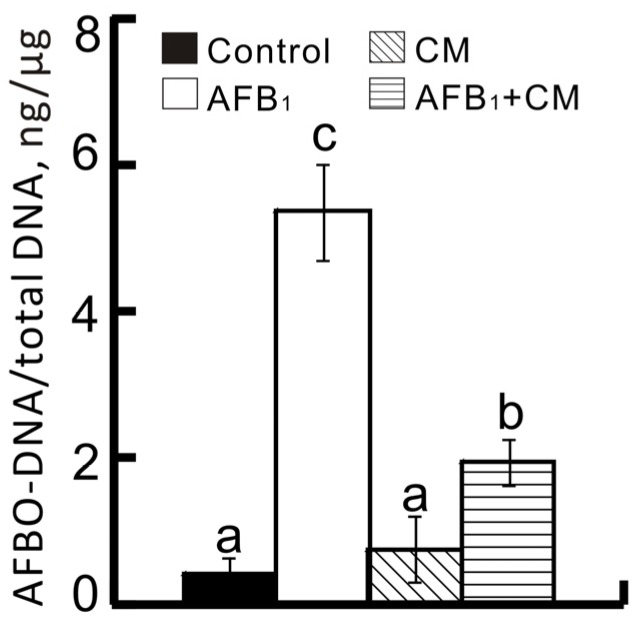
The concentrations of AFBO–DNA adducts in the liver were significantly affected by either supplementation of AFB1 or CM at week 2 (Figure 2). Compared to the control, the hepatic AFBO–DNA adduct content was increased (p < 0.05) 12 times by AFB1 supplementation. Interestingly, the AFB1 + CM group decreased (p < 0.05) the concentration of AFBO–DNA adduct (63.7%) in the liver when compared to the AFB1 group.
Figure 2.
Effects of dietary AFB1 and CM concentrations on the contents of AFBO–DNA adducts in the liver of chicks at week 2. Values are expressed as means ± SD (n = 5), and means with different superscript letters differ (p < 0.05). AFB1, aflatoxin B1; AFBO, exo-AFB1-8,9-epoxide; CM, curcumin. Experimental details of Control and AFB1 groups are given in Sun et al. (2016) [12].
2.4. Hepatic CYP450 Isozyme Activities and Gene Expression
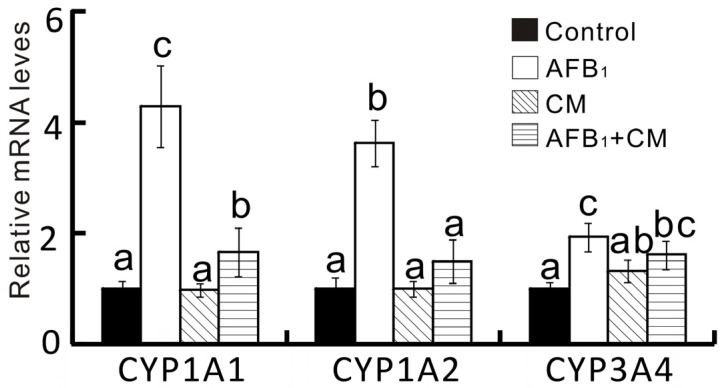
The mRNA levels of CYP1A1, CYP1A2, and CYP3A4 in the liver were significantly altered by either supplementation of AFB1 or CM (Figure 3). Specifically, dietary AFB1 supplementation led to upregulated (p < 0.05) mRNA levels of CYP1A1, CYP1A2, and CYP3A4 in liver microsomes. Strikingly, the increased hepatic CYP450 isozyme mRNA levels observed in the AFB1 group were suppressed in the AFB1 + CM group. It is fascinating to find that the effects of AFB1 and CM on changes in hepatic CYP450 isozyme mRNA levels were in parallel with their activities.
Figure 3.
Effects of dietary AFB1 and CM concentrations on relative mRNA abundance of CYP450 isozyme genes in liver of chicks at week 2. Values are expressed as means ± SD (n = 5), and means with different superscript letters differ (p < 0.05). AFB1, aflatoxin B1; CM, curcumin; CYP1A1, Cytochrome P450 1A1; CYP1A2, Cytochrome P450 1A2; CYP3A4, Cytochrome P450 3A4. Experimental details of Control and AFB1 groups are given in Sun et al. (2016) [12].
3. Discussion
Protection against AFB1-induced hepatotoxic effects was successfully replicated in broiler chicks fed an AFB1-contaminated corn–soybean diet with CM supplementation. Although the dietary AFB1 had no significant effect on growth performance in chicks, it induced the typical clinical signs of hepatic injury, including increased activities of AST and ALT, decreased concentrations of ALB and TP in serum, as well as bile duct hyperplasia and necrosis in the liver of chicks at week 2 [28,29]. However, the serological results indicated that AFB1-induced liver injury disappeared at week 4. The reasons for this might be that older poultry was more resistant to aflatoxicosis than young poultry [30], and the AFBO–DNA adducts could be repaired by the nucleotide excision repair system in liver [31]. Intriguingly, dietary supplementation of CM mitigated serum and histopathological parameter alterations that were induced by dietary supplementation of AFB1 at week 2. These outcomes were consistent with previous studies, which provided evidence that hepatic injury was induced by dietary AFB1 as well as that dietary CM supplementation displayed protective effects against its negative effects [15,19,20,21]. Moreover, the present study displayed AFB1-induced oxidative stress in chickens as evidenced by decreased antioxidant ability (GPX, CAT, and GSH), increased lipid peroxidation (MDA), and DNA damage (8-OHdG). On the other hand, dietary CM supplementation inhibited these changes. Meanwhile, dietary supplementation of CM alone improved the hepatic GPX activity, which was consistent with previous studies in which CM increased GPX activity, probably by activating the Nrf2–keap1 pathway [32,33]. A previous study showed that dietary supplementation of 5000 mg/kg CM increased hepatic phase II detoxification enzyme (GST) activity in rats that were exposed to AFB1 [34], while GST activity was not affected by CM in this study. The divergence between these reports could be attributed to the different animal species and ingestion dose. Taken together, these outcomes are similar to former studies, which reported that oxidative stress could be due to the direct effects of AFB1, its metabolites, and/or the generation of free radicals [11,35]. Dietary supplementation of CM, however, showed protective actions against AFB1-induced hepatic injury, which were associated with the enhancement of antioxidant capacities [18,19,21,22].
The most interesting finding from the present study was that the four major CYP450 isozymes were significantly inhibited to a large extent by dietary supplementation of CM upon exposure to dietary AFB1. The hepatic mRNA levels and/or enzyme activities of CYP1A1, CYP1A2, CYP2A6, and CYP3A4 were significantly increased when chicks were exposed to dietary AFB1, while dietary supplementation of CM inhibited these changes. Because a previous study reported that CYP2A6 and (to a lesser extent) CYP1A1 are responsible for the bioactivation of AFB1 into AFBO in chicken hepatic microsomes, and that CYP1A2 and CYP3A4 are the most important enzymes capable of bioactivating AFB1 into AFBO in mammals [23,36], inhibition of the activities of these enzymes could decrease the production of AFBO. Indeed, as a major toxic adduct of AFBO [10,36], the AFBO–DNA was sharply decreased by the dietary supplementation of CM when chicks were exposed to dietary AFB1. These findings suggest that the protective actions of CM may be mediated through inhibited activities of these crucial CYP450 isozymes, which could decrease the production of the highly toxic AFBO. These outcomes are in agreement with previous studies, which showed that CM-mediated inhibition of AFB1 toxicity was associated with a reduction of the formation of AFBO–DNA by modulating CYP450 function, including CYP1A1 activity [14,20], while the activities of CYP1A2, CYP2A6, and CYP3A4 inhibited by CM in this process are described, to our knowledge, for the first time in the current study. Similarly, CM has been shown to inhibit tumorigenesis induced by benzo(a)pyrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin through the inhibition of CYP1A1, CYP1B1, and/or CYP1A2 enzyme on mRNA, protein, and/or activities levels [25,37].
The CYP450s are important metabolizing enzymes, most of which are mainly expressed in the liver. Clinically, these enzymes play vital roles in drug metabolism and are required for the efficient clearance of xenobiotics from the body [38,39]. On the other hand, these enzymes can also bioactivate biologically inert carcinogens and toxins, such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone [40], N-nitrosonornicotine [41], hexamethylphosphoramide [42], benzo(a)pyrene [25], AFB1 [10], and 2,6-dichlorobenzonitrile [43] to electrophilic metabolites that can cause toxicity, cell death, and sometimes cellular transformation that results in cancer. Given that CM is a strong inhibitor of CYP450 isozymes, it could be a potentially promising chemopreventive agent for many carcinogens and toxins, even though its mechanism of action remains to be clarified.
In summary, the present study successfully confirmed that dietary CM supplementation could alleviate AFB1-induced liver injury, with regard to the suppression of serum biochemistry changes and histopathological lesions in the liver of broilers. The protective mechanism of CM against AFB1-induced adverse effects may be associated with: (1) the common mechanism that CM could reduce AFB1-induced oxidative stress by increasing antioxidant capacities; and (2) the novel finding that CM could effectively inhibit the regulatory role of CYP450 isozymes that are crucial for the activation of AFB1 to highly toxic AFBO. Further validation of the potential mechanisms of the interactions between CM and the CYP450 isozymes would be beneficial to gain a better understanding of the detoxification mechanism of CM and applications of CM to control chemical carcinogenesis.
4. Materials and Methods
4.1. Chicks, Treatments, and Samples Collection
Our animal protocol was approved by the Scientific Ethic Committee of Huazhong Agricultural University on 8 August 2015. The project identification code is HZAUCH-2015-007. In total, 120 one-day-old male avian broilers were randomly divided into four treatment groups with five replicates of six chicks per pen. All chicks were allowed free access to a corn/soybean-based diet (BD) formulated to meet the nutritional requirements of broilers’ diets (Table S2) as reported previously [12], and distilled water. After three days of acclimation, the four experimental groups were arranged in a two by two factorial design trial that included BD diet supplemented with AFB1 (Sigma-Aldrich, St. Louis, MO, USA) and CM (China National Medicines Corp. Ltd., Beijing, China), as follows: Control; AFB1 (100 µg AFB1/kg); CM (150 mg CM/kg); and AFB1 + CM (100 µg AFB1/kg with 150 mg CM/kg). The experiment lasted for four weeks. The doses were chosen based on previous studies, which reported that dietary consumption of approximately 100 μg AFB1/kg induced adverse effects [44], while dietary supplementation of 74–222 mg CM/kg displayed a protective effect on AFB1 in broilers [19]. Individual body weights and feed intake of broilers were measured biweekly. Meanwhile, chicks (n = 5/group) were euthanized by decapitation to collect blood and livers for the preparation of serum and liver histological tissue samples as previously described [29].
4.2. Dietary AFB1 Analysis, and Feed Preparation
Twenty grams of moldy corn was extracted with 100 mL of methanol (Fisher, Pittsburgh, PA, USA):water (70:30, v/v) for AFB1 detection. After shaking for 3 min, the supernatant of the extract was filtered through a Whatman filter (Whatman, Clifton, NJ, USA), and the filtrate was collected. Then, the concentration of AFB1 in filtrate was measured followed the protocol of the ELISA kit (AgraQuant® Aflatoxin B1 Assay, Romer, Singapore). The powdered feed was mixed with a vertical mixer.
4.3. Serum Biochemical and Histological Analysis
The serum activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), along with concentrations of albumin (ALB) and total protein (TP) were determined in serum samples. Analysis of the serum samples was measured by an automatic biochemistry analyzer (Beckman Synchron CX4 PRO, Fullerton, CA, USA). The liver tissues were fixed in 10% neutral buffered formalin and processed for paraffin embedding, sectioned at 5 µm, and then stained with hematoxylin and eosin, by standard procedure [29]. Liver sections from all broilers were microscopically examined.
4.4. Antioxidant Enzyme Activities Analysis
Liver samples (0.5 g) were thawed in 4.5 mL isotonic saline on ice and homogenized as previously described [33]. The supernatants were then prepared by centrifugation at 12,000 × g for 15 min at 4 °C. Activities of superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and GST, as well as concentrations of GSH and malondialdehyde (MDA) were determined using the colorimetric method through the specific assay kits (A001, A005, A007-1, A004 A006-1 and A003), which were purchased from the Nanjing Jiancheng Bioengineering Institute of China. The concentration of 8-hydroxydeoxyguanosine (8-OHdG) in serum was measured using the ELISA kit (H165, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Concentrations of protein were determined using the bicinchoninic acid assay [45].
4.5. Hepatic AFBO–DNA Adduct Analysis
Liver genomic DNA was extracted using the DNA extraction kit following the manufacturer’s instructions (Qiagen, Shanghai, China). The DNA concentrations were quantified by the 260/280 nm absorbance ratio by an Agilent Bioanalyzer 2100 (Agilent Technologies, Amstelveen, The Netherlands). Genomic DNA (15 μg) was used to determine the AFBO–DNA adduct amount using a competitive ELISA method, according to the manufacturer’s instructions (Cell Biolabs, Inc., San Diego, CA, USA).
4.6. Hepatic Microsomal CYP450 Isozyme Activities Analysis
The liver microsomes and cytosolic fractions were prepared as described previously [46]. The microsomal activities of 7-ethoxyresorufin-O-deethylase, methoxyresorufin-O-demethylase, coumarin 7-hydroxylase, and nifedipine oxidation were determined to assess chicken orthologs of human CYP1A1, CYP1A2, CYP2A6, and CYP3A4 activities, respectively [23]. The concentrations of protein were measured as described above [45].
4.7. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was extracted from liver samples by using Trizol (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The quality and quantity of the RNA samples were analyzed by an Agilent Bioanalyzer 2100 using an RNA 6000 Labchip kit (Agilent Technologies, Amstelveen, The Netherlands). The cDNA was synthesized from 1.0 μg total RNA by using Super Script III reverse transcriptase (Invitrogen). The mRNA levels of CYP450 isozyme genes were determined by qPCR (7900 HT; Applied Biosystems, Foster City, CA, USA). The primers of CYP450 isozymes and reference gene glyceraldehyde-3-phosphate dehydrogenase were reported previously [12]. The 2−ddCt method was used for the quantification, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene and the relative abundance normalized to the control (as 1) [47].
4.8. Statistical Analysis
One-way ANOVA followed by Fisher’s least significant difference (LSD) test was used to compare the effects between groups on each variable within the same tissue. Data are presented as means ± SD, and significance level was set at p < 0.05. All analyses were conducted using SAS 8.2 (SAS Institute, Cary, NC, USA).
Acknowledgments
This project was supported by the Chinese Natural Science Foundation projects (31501987 and 31272479), National Key Research and Development Program of China (2016YFD0501207). We thank the Journal of Nutrition for the use of data of Control and AFB1 groups that were originally published therein.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/8/11/327/s1, Table S1: Effects of dietary AFB1 and CM concentrations on growth performance in chicks, Table S2: Basal diet formulations and nutritional contents.
Author Contributions
L.-H.S. designed the research; N.-Y.Z., M.Q., L.Z., M.-K.Z., J.G., J.L. and C.-Q.G. conducted the experiments and analyzed the data; L.-H.S., N.-Y.Z., S.A.R., C.S.K. and D.-S.Q. wrote the paper; and L.-H.S. had primary responsibility for the final content.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Koehler P.E., Hanlin R.T., Beraha L. Production of aflatoxins B1 and G1 by Aspergillus flavus and Aspergillus parasiticus isolated from market pecans. Appl. Microbiol. 1975;30:581–583. doi: 10.1128/am.30.4.581-583.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L.H., Lei M.Y., Zhang N.Y., Zhao L., Krumm C.S., Qi D.S. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem. Toxicol. 2014;74:289–293. doi: 10.1016/j.fct.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Hussein H.S., Brasel J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167:101–134. doi: 10.1016/S0300-483X(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 4.Monson M.S., Settlage R.E., McMahon K.W., Mendoza K.M., Rawal S., El-Nezami H.S., Coulombe R.A., Reed K.M. Response of the hepatic transcriptome to aflatoxin B1 in domestic turkey (Meleagris gallopavo) PLoS ONE. 2014;9:e100930. doi: 10.1371/journal.pone.0100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rustemeyer S.M., Lamberson W.R., Ledoux D.R., Wells K., Austin K.J., Cammack K.M. Effects of dietary aflatoxin on the hepatic expression of apoptosis genes in growing barrows. J. Anim. Sci. 2011;89:916–925. doi: 10.2527/jas.2010-3473. [DOI] [PubMed] [Google Scholar]
- 6.Yarru L.P., Settivari R.S., Gowda N.K., Antoniou E., Ledoux D.R., Rottinghaus G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009;88:2620–2627. doi: 10.3382/ps.2009-00204. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans—Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs, Volumes 1–42. IARC; Lyon, France: 1987. [Google Scholar]
- 8.Wu F., Guclu H. Aflatoxin regulations in a network of global maize trade. PLoS ONE. 2012;7:e45151. doi: 10.1371/journal.pone.0045151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zain M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011;15:129–144. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- 10.Yunus A.W., Razzazi-Fazeli E., Bohm J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins (Basel) 2011;3:566–590. doi: 10.3390/toxins3060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mary V.S., Theumer M.G., Arias S.L., Rubinstein H.R. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology. 2012;302:299–307. doi: 10.1016/j.tox.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Sun L.H., Zhang N.Y., Zhu M.K., Zhao L., Zhou J.C., Qi D.S. Prevention of Alfatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome P450 isozymes and up-regulation of six selenoprotein genes in chick liver. J. Nutr. 2016;143:1115–1122. doi: 10.3945/jn.113.177410. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 14.Firozi P.F., Aboobaker V.S., Bhattacharya R.K. Action of curcumin on the cytochrome P450-system catalyzing the activation of aflatoxin B1. Chem. Biol. Interact. 1996;100:41–51. doi: 10.1016/0009-2797(95)03684-9. [DOI] [PubMed] [Google Scholar]
- 15.Poapolathep S., Imsilp K., Machii K., Kumagai S., Poapolathep A. The effects of curcumin on aflatoxin B1-induced toxicity in rats. Biocontrol. Sci. 2015;20:171–177. doi: 10.4265/bio.20.171. [DOI] [PubMed] [Google Scholar]
- 16.Satoskar R.R., Shah S.J., Shenoy S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986;24:651–654. [PubMed] [Google Scholar]
- 17.Smith W.A., Freeman J.W., Gupta R.C. Effect of chemopreventive agents on DNA adduction induced by the potent mammary carcinogen dibenzo[a,l]pyrene in the human breast cells MCF-7. Mutat. Res. 2001;480–481:97–108. doi: 10.1016/S0027-5107(01)00173-7. [DOI] [PubMed] [Google Scholar]
- 18.El-Bahr S.M. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin B1. Phytother. Res. 2015;29:134–140. doi: 10.1002/ptr.5239. [DOI] [PubMed] [Google Scholar]
- 19.Gowda N.K., Ledoux D.R., Rottinghaus G.E., Bermudez A.J., Chen Y.C. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 2009;102:1629–1634. doi: 10.1017/S0007114509990869. [DOI] [PubMed] [Google Scholar]
- 20.Nayak S., Sashidhar R.B. Metabolic intervention of aflatoxin B1 toxicity by curcumin. J. Ethnopharmacol. 2010;127:641–644. doi: 10.1016/j.jep.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 21.El-Agamy D.S. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats. Arch. Toxicol. 2010;84:389–396. doi: 10.1007/s00204-010-0511-2. [DOI] [PubMed] [Google Scholar]
- 22.Verma R.J., Mathuria N. Curcumin ameliorates aflatoxin-induced lipid-peroxidation in liver and kidney of mice. Acta Pol. Pharm. 2008;65:195–202. [PubMed] [Google Scholar]
- 23.Diaz G.J., Murcia H.W., Cepeda S.M. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010;89:2461–2469. doi: 10.3382/ps.2010-00864. [DOI] [PubMed] [Google Scholar]
- 24.Appiah-Opong R., Commandeur J.N., van Vugt-Lussenburg B., Vermeulen N.P. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology. 2007;235:83–91. doi: 10.1016/j.tox.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Garg R., Gupta S., Maru G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh Y.W., Huang C.Y., Yang S.Y., Peng Y.H., Yu C.P., Chao P.D., Hou Y.C. Oral intake of curcumin markedly activated CYP 3A4: In vivo and ex vivo studies. Sci. Rep. 2014;4:6587. doi: 10.1038/srep06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thapliyal R., Maru G.B. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food Chem. Toxicol. 2001;39:541–547. doi: 10.1016/S0278-6915(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 28.Bagherzadeh Kasmani F., Karimi Torshizi M.A., Allameh A., Shariatmadari F. A novel aflatoxin-binding Bacillus probiotic: Performance, serum biochemistry, and immunological parameters in Japanese quail. Poult. Sci. 2012;91:1846–1853. doi: 10.3382/ps.2011-01830. [DOI] [PubMed] [Google Scholar]
- 29.Sun L.H., Zhang N.Y., Sun R.R., Gao X., Gu C., Krumm C.S., Qi D.S. A novel strain of Cellulosimicrobium funkei can biologically detoxify aflatoxin B1 in ducklings. Microb. Biotechnol. 2015;8:490–498. doi: 10.1111/1751-7915.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein P.J., Van Vleet T.R., Hall J.O., Coulombe R.A., Jr. Biochemical factors underlying the age-related sensitivity of turkeys to aflatoxin B1. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002;132:193–201. doi: 10.1016/S1532-0456(02)00065-0. [DOI] [PubMed] [Google Scholar]
- 31.Bedard L.L., Massey T.E. Aflatoxin B1-induced DNA damage and its repair. Cancer Lett. 2006;241:174–183. doi: 10.1016/j.canlet.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Piper J.T., Singhal S.S., Salameh M.S., Torman R.T., Awasthi Y.C., Awasthi S. Mechanisms of anticarcinogenic properties of curcumin: The effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int. J. Biochem. Cell Biol. 1998;30:445–456. doi: 10.1016/S1357-2725(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 33.Soetikno V., Sari F.R., Lakshmanan A.P., Arumugam S., Harima M., Suzuki K., Kawachi H., Watanabe K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol. Nutr. Food Res. 2013;57:1649–1659. doi: 10.1002/mnfr.201200540. [DOI] [PubMed] [Google Scholar]
- 34.Kelly V.P., Ellis E.M., Manson M.M., Chanas S.A., Moffat G.J., McLeod R., Judah D.J., Neal G.E., Hayes J.D. Chemoprevention of aflatoxin B1 hepatocarcinogenesis by coumarin, a natural benzopyrone that is a potent inducer of aflatoxin B1-aldehyde reductase, the glutathione S-transferase A5 and P1 subunits, and NAD(P)H: Quinone oxidoreductase in rat liver. Cancer Res. 2000;60:957–969. [PubMed] [Google Scholar]
- 35.Kanbur M., Eraslan G., Sarıca Z.S., Aslan O. The effects of evening primrose oil on lipid peroxidation induced by subacute aflatoxin exposure in mice. Food Chem. Toxicol. 2011;49:1960–1964. doi: 10.1016/j.fct.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Guengerich F.P., Johnson W.W., Shimada T., Ueng Y.F., Yamazaki H., Langouët S. Activation and detoxication of aflatoxin B1. Mutat. Res. 1998;402:121–128. doi: 10.1016/S0027-5107(97)00289-3. [DOI] [PubMed] [Google Scholar]
- 37.Choi H., Chun Y.S., Shin Y.J., Ye S.K., Kim M.S., Park J.W. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci. 2008;99:2518–2524. doi: 10.1111/j.1349-7006.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anzenbacher P., Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell Mol. Life Sci. 2001;58:737–747. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Smith T.J., Stoner G.D., Yang C.S. Activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human lung microsomes by cytochromes P450, lipoxygenase, and hydroperoxides. Cancer Res. 1995;55:5566–5573. [PubMed] [Google Scholar]
- 41.Patten C.J., Smith T.J., Friesen M.J., Tynes R.E., Yang C.S., Murphy S.E. Evidence for cytochrome P450 2A6 and 3A4 as major catalysts for N9-nitrosonornicotine a-hydroxylation by human liver microsomes. Carcinogenesis (Lond.) 1997;18:1623–1630. doi: 10.1093/carcin/18.8.1623. [DOI] [PubMed] [Google Scholar]
- 42.Su T., Bao Z., Zhang Q.Y., Smith T.J., Hong J.Y., Ding X. Human cytochrome P450 CYP2A13: Predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- 43.Xie F., D’Agostino J., Zhou X., Ding X. Bioactivation of the nasal toxicant 2,6-dichlorobenzonitrile: An assessment of metabolic activity in human nasal mucosa and identification of indicators of exposure and potential toxicity. Chem. Res. Toxicol. 2013;26:388–398. doi: 10.1021/tx300479w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J., Bai F., Zhang K., Bai S., Peng X., Ding X., Li Y., Zhang J., Zhao L. Effects of feeding corn naturally contaminated with aflatoxin B1 and B2 on hepatic functions of broilers. Poult. Sci. 2012;91:2792–2801. doi: 10.3382/ps.2012-02544. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y., Zhao H., Zhang Q., Tang J., Li K., Xia X.J., Wang K.N., Li K., Lei X.G. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J. Nutr. 2012;142:1410–1416. doi: 10.3945/jn.112.159020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein P.J., Buckner R., Kelly J., Coulombe R.A., Jr. Biochemical basis for the extreme sensitivity of turkeys to aflatoxin B1. Toxicol. Appl. Pharmacol. 2000;165:45–52. doi: 10.1006/taap.2000.8926. [DOI] [PubMed] [Google Scholar]
- 47.Sun L.H., Li J.G., Zhao H., Shi J., Huang J.Q., Wang K.N., Xia X.J., Li L., Lei X.G. Porcine serum can be biofortified with selenium to inhibit proliferation of three types of human cancer cells. J. Nutr. 2013;143:1115–1122. doi: 10.3945/jn.113.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.