Table 5.
| racemic substrate | entry | solvent | k rel |
|---|---|---|---|
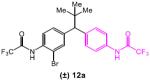
|
1 | DMF/Tol | 5.3 |
| 2 | DMF | 3.5 | |
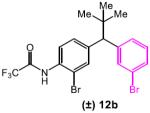
|
3 | DMF/Tol | 11.3 |
| 4 | DMF | 6.9 | |
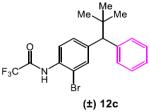
|
5 | DMF/Tol | 10.3 |
| 6 | DMF | 4.8 |
Reaction conditions: racemic substrate (0.3 mmol), diethyl malonate (1.1 equiv), Cu(MeCN)4BF4 (5 mol%), peptide (10 mol%), Cs2CO3 (3.2 equiv), DMF/Tol (0.38/0.83 mL) or DMF (1.2 mL).
Conversions were determined by 1H NMR and enantiomeric ratios were determined by chiral high performance liquid chromatography analysis. See supporting information for details. Results are reported as an average of two runs.

