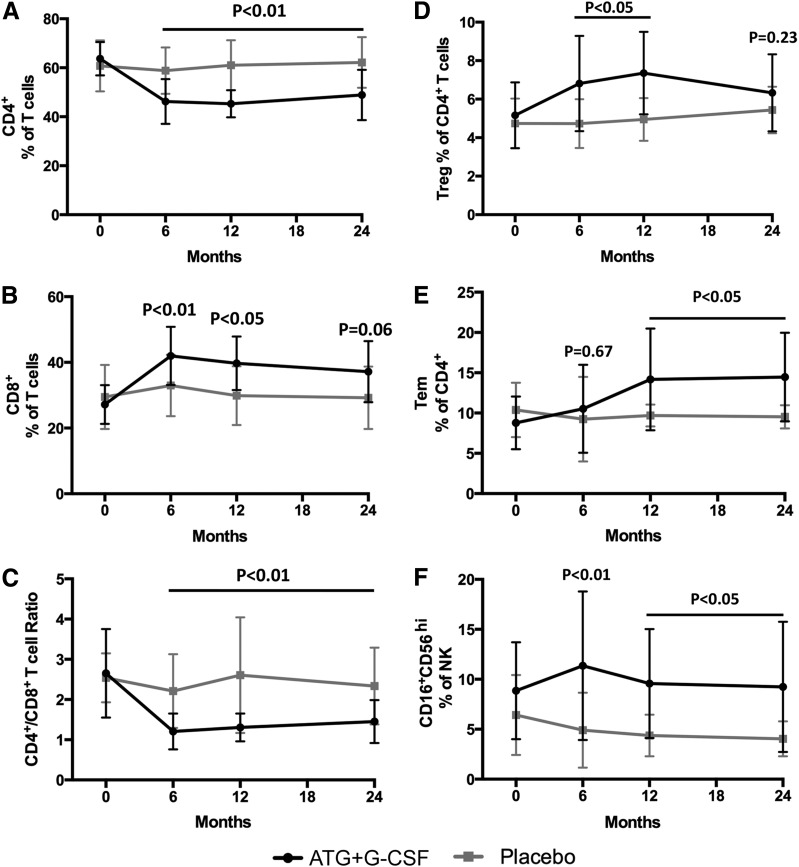
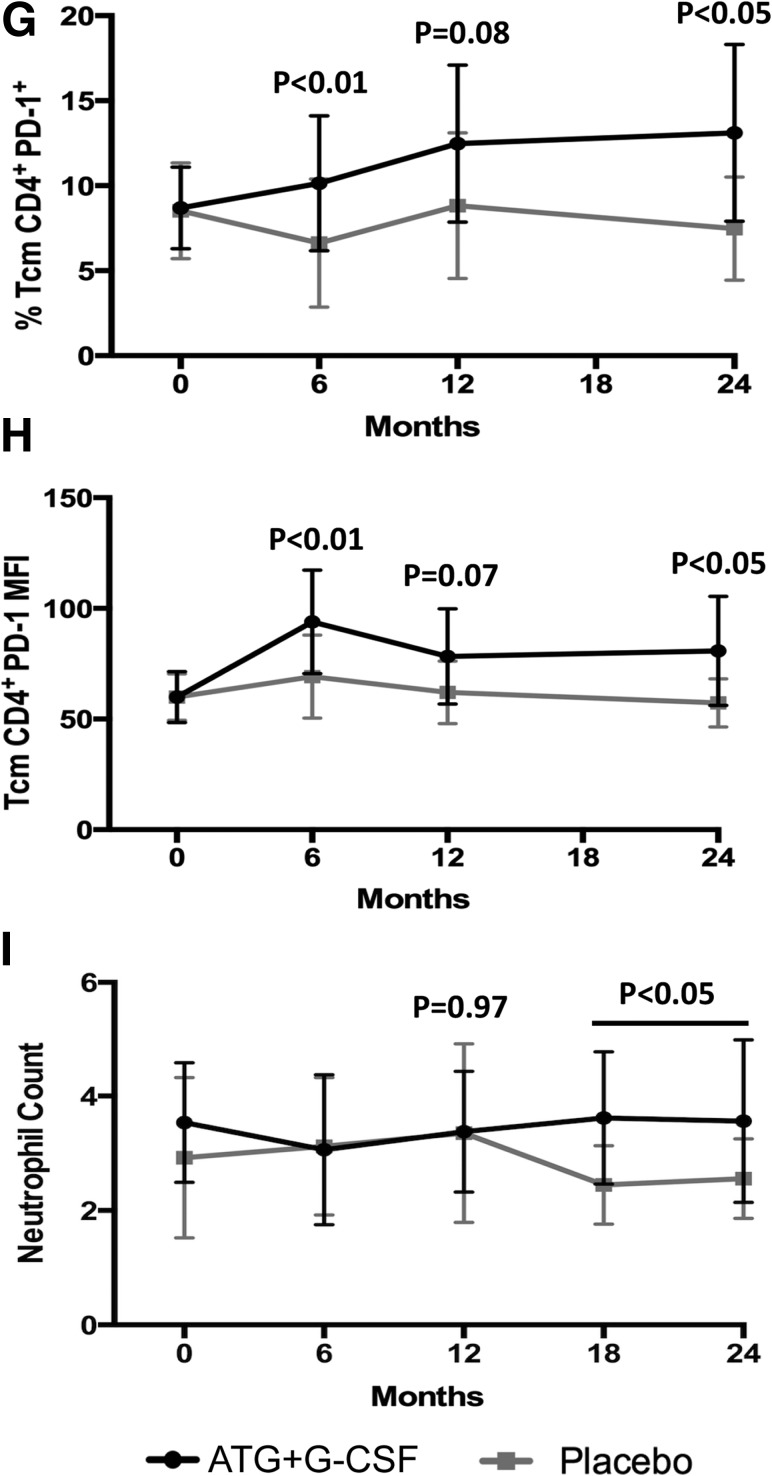
Figure 2.
Immunomodulatory effects of ATG+G-CSF combination therapy remain detectable at least 24 months following treatment. PBMCs were characterized by flow cytometric analysis, and complete blood count was performed. Compared with placebo (gray squares; N = 8), subjects treated with ATG+G-CSF (black circles; N = 16) exhibited reduced CD4+ T-cell frequency (A), increased CD8+ T-cell frequency (B), and reduced CD4+/CD8+ T-cell ratio (C). D: CD4+CD25+FOXP3+Helios+ Tregs were significantly elevated in ATG+G-CSF–treated subjects at 6 and 12 months, but the difference was not significant at 24 months. CD4+ Tem frequency (E), CD16+CD56hi NK percent (F), CD4+PD-1+ Tcm frequency (G), PD-1 MFI on CD4+ Tcm (H), and neutrophil counts (I) were significantly greater in ATG+G-CSF–treated subjects compared with control subjects. All P values shown are for ATG+G-CSF vs. placebo at the indicated time points.