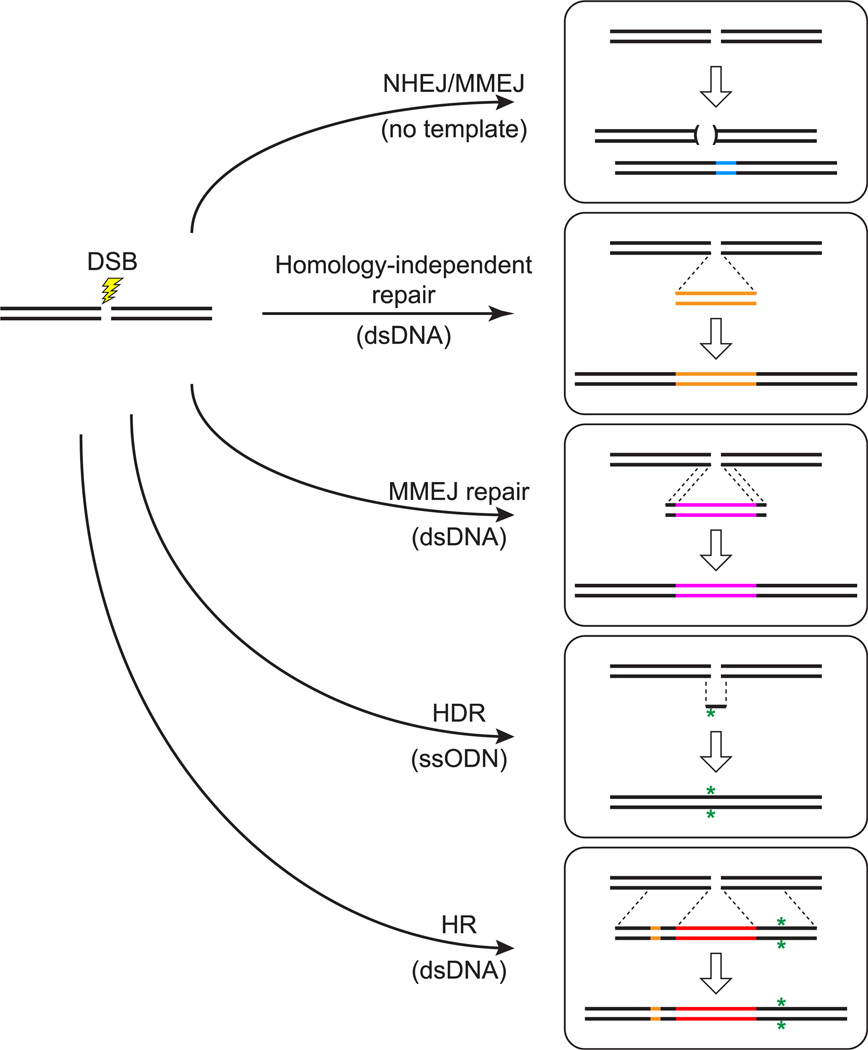
FIGURE 1. Approaches to genome editing.
Repair and recombination events are stimulated by double-strand breaks (DSBs) induced by targeted cleavage (lightning bolt) of the genome with programmable nucleases. In the absence of template DNA to guide repair, the nonhomologous end-joining (NHEJ) or microhomology-mediated end-joining (MMEJ) pathways heal broken ends of chromosomes in a process that often generates small deletions and/or insertions at the site of the lesion. These repair pathways can also facilitate integration of exogenously supplied DNA sequences at the lesion site. In the presence of linear double-stranded DNA (dsDNA) donor molecules that bear no homology to the targeted locus, the NHEJ pathway may join donor DNA sequences to the broken chromosome ends. End-joining occurs in a homology-independent, imprecise manner, and foreign sequences may be integrated in either orientation. If the borders of the donor DNA contain short sequences homologous to the regions immediately flanking the DSB site, end resection may uncover short stretches of complementarity that guide repair and result in incorporation of the donor sequences at the lesion. Two additional methods have been employed with the purpose of precisely modifying the host genome. When single-stranded oligodeoxynucleotides (ssODN) with close homology to the site of the DSB are available, the donor sequences may guide homology-directed repair (HDR) resulting in the modification of host sequences. Finally, when DSBs are induced in the presence of dsDNA molecules with extensive homology to the targeted region, true crossover events mediated by homologous recombination (HR) may occur, resulting in exchange of several kilobase-long regions between the host genome and the exogenously supplied DNA. This approach can be used to produce several closely linked genome modifications simultaneously. (See color plate)