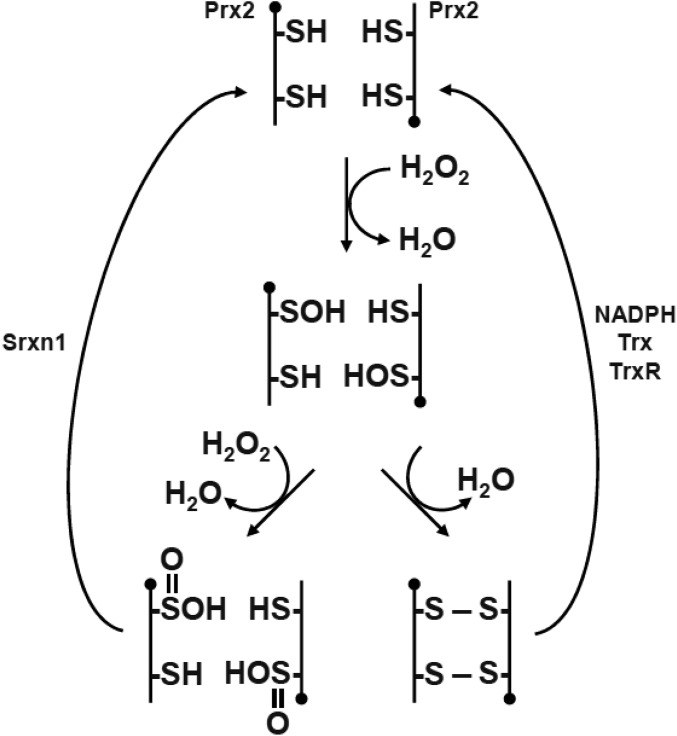
Fig. S1.
Catalytic cycle of Prx2. The peroxidatic cysteine thiol (-SH) of active Prx2 can reduce H2O2 to form a sulfenic acid (-SOH). In turn, this adduct either can be reduced by another active Prx2 to form an intermolecular disulfide bond (S—S) or can be overoxidized to produce sulfinic acid (-SO2H). Both the disulfide bond and overoxidized form can be regenerated to their active states by coordinated action of the thioredoxin (Trx) pathway [NADPH, thioredoxin, and thioredoxin reductase (TrxR)] or by the reductase Srxn1.