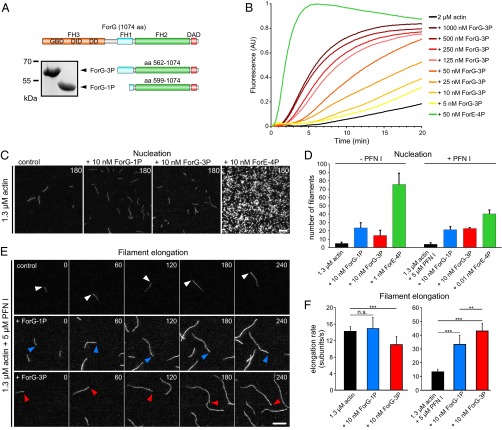
Fig. 5.
ForG promotes F-actin assembly. (A) Domain organization of ForG and derived C-terminal constructs used for biochemical analyses. (Inset) Purified ForG-1P and ForG-3P fragments. (B) ForG-3P promoted polymerization of pyrene-labeled G-actin (2 μM, 5% labeled) in a concentration-dependent manner. Note markedly higher assembly rates by 50 nM ForE-4P. AU, arbitrary units. (C) ForG exhibits rather weak actin filament nucleation compared with ForE-4P. Representative frames from TIRFM time-lapse recordings of actin filament assembly are shown. (D) Nucleation efficacies of ForG and ForE constructs in the absence or presence of PFN I (mean ± SD, n = 3). Note that due to the strong nucleation efficacy, and for technical reasons, 10- or 1,000-fold less of ForE-4P had to be used for the quantifications. (E) Filament elongation visualized by TIRFM in the presence of 5 μM PFN I alone or in combination with either 10 nM ForG-1P or 10 nM ForG-3P. Barbed ends are marked by arrowheads. Time is given in seconds. (F) Elongation rates of growing filaments were calculated from time-lapse movies as shown in E. (Left) In the absence of PFN I, addition of 10 nM ForG-3P led only to slightly reduced elongation compared with rates of control and ForG-1P. (Right) In the presence of PFN I, ForG-1P– and ForG-3P–associated filaments were elongated about threefold faster than control filaments (mean ± SD). At least 20 individual filaments were tracked. ***P ≤ 0.001, **P ≤ 0.01 (Mann-Whitney U test). Note that the scale differs between the left and right graphs. (Scale bars: C, 5 μm; E, 10 μm.)