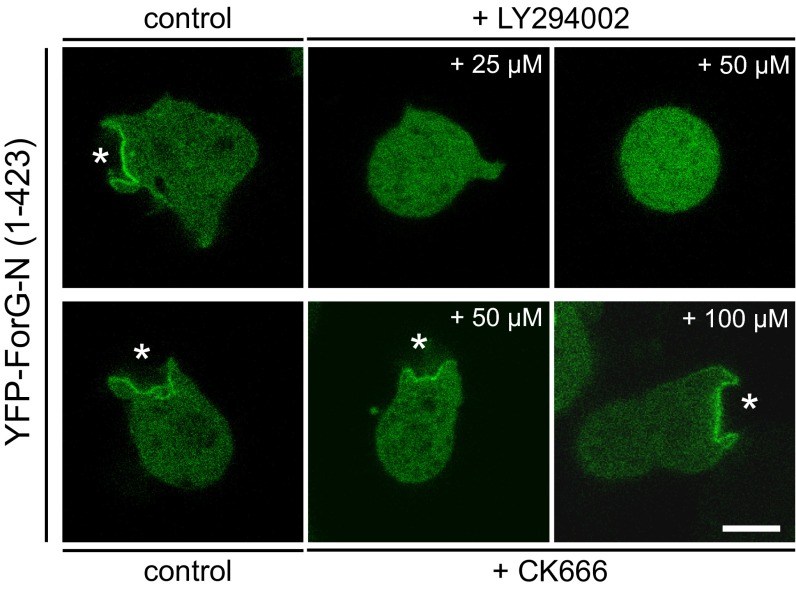
Fig. S7.
Recruitment of ForG-N is PIP3- but not Arp2/3 complex-dependent. Vegetative WT cells expressing YFP–ForG-N were seeded on glass-bottom dishes in low-osmolarity phosphate buffer and imaged either in the absence or presence of the inhibitors indicated. Notably, treatment of cells with the PIP3 kinase inhibitor LY2904002 at 25 μM or 50 μM was sufficient to delocalize ForG-N completely from the membrane and render it entirely cytosolic, supporting the view that PIP3, together with Ras signaling, is required for ForG targeting (also Fig. 6E). By contrast, treatment with the Arp2/3 complex inhibitor CK666 showed no significant effect at 50 μM and 100 μM, indicating that ForG does not require the Arp2/3 complex for targeting to the cups (white stars). Confocal sections of live cells are shown. (Scale bar: 5 μm.)