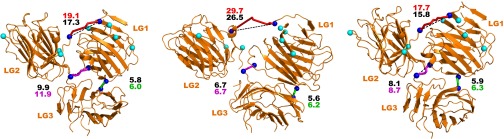
Fig. S4.
Three possible structures of LG1, LG2, and LG3 domains as suggested by molecular docking under constraints from observed cross-links. Cβ atoms of linked side chains for cross-links between the LG domains are indicated by blue spheres (Cα for glycine). Residues found to be linked to the carboxyl-terminal region of the coiled coil are indicated by cyan spheres. Cross-links were mapped by homology onto the structure of the α2 LG domains (extracted from PDB ID code 2WJS). Solvent accessible surface distances between Cβ atoms of linked residues are shown as a magenta tube (SDH link), green tube (zero-length link), or red tube (polypeptide link). Direct Cβ-Cβ distances are indicated by dashed lines. Distances in Ångstrom calculated according to the two routes are shown for each cross-link in the appropriate color.