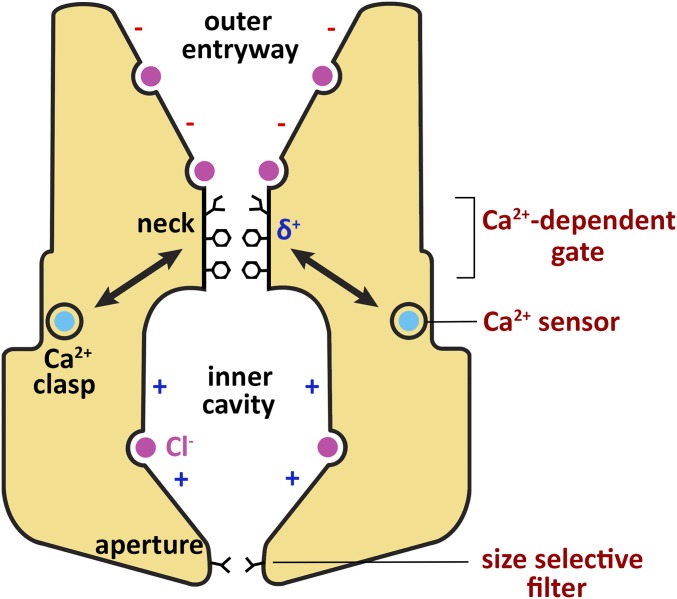
Fig. 8.
Regions of BEST1 involved in Ca2+-dependent activation and ion selectivity. Structural features identified from the X-ray structure of BEST1 are labeled in black (16), and the mechanistic functions of these regions identified in this study are labeled in red (Right). Binding of intracellular Ca2+ (teal) to the Ca2+ clasp, which acts as a Ca2+ sensor, is coupled to conformational changes at the neck, which functions as a Ca2+-dependent gate. The neck is lined by three conserved hydrophobic residues (I76, F80, F84, stick representations). Phenylalanine residues within the neck (F80 and F84) may contribute favorable anion–quadropole interactions (δ+) to traversing Cl− ions, but these interactions do not determine ion selectivity. The cytosolic aperture, which is lined by the V205 residue, acts as a size-selective filter and endows BEST1 with its characteristic lyotropic permeability sequence. Selectivity for Cl− and other monovalent anions over cations may be determined by anion binding sites observed in the pore of BEST1 (purple) (16). The minus (−) and plus (+) signs in the pore indicate that, aside from the anion binding sites, the walls of the outer entryway and inner cavity have predominately negative and positive electrostatic potentials, respectively.