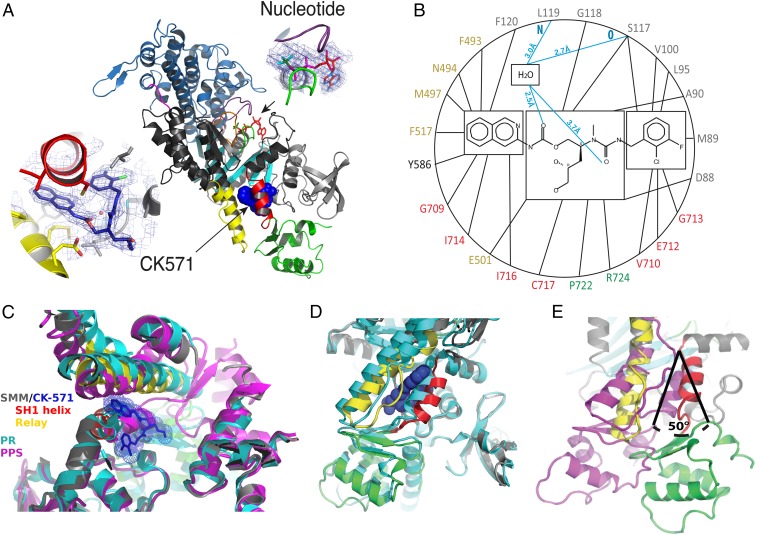
Fig. 2.
An allosteric pocket opens to bind CK-571 during the recovery stroke. (A) Overall view of the myosin MD of the SMM/CK-571 structure in cartoon representation. CK-571 is shown in blue spheres and is located 22 Å away from the nucleotide (Right Inset: red sticks, ADP; green sticks, BeFx). CK-571 (Left Inset: 2Fo-Fc electron density) interacts with residues of the SH1 helix (red), Relay (yellow), and the Nter subdomain (light gray) as well as with Y586 from the L50 subdomain (dark gray). The other two subdomains of the motor are the U50 (blue) and converter (green). (B) Residues of the CK-571 binding site (black lines, apolar contacts; blue lines, polar contacts). Residues are indicated with the same color code as in A. (C) Comparison of the CK-571 binding pocket with the myosin PR (PDB ID code 3I5F, cyan) and PPS (PDB ID code 1BR1, magenta) states. Only the SH1 helix and the relay conformation in the SMM/CK-571 structure are compatible with the drug binding. (D) Comparison of the SMM/CK-571 structure with the myosin PR state (PDB ID code 3I5F, cyan). In the SMM/CK-571 structure, rearrangements of the Relay (yellow) and a 10° rotation of the SH1 helix (red) allow the formation of a cleft in the molecule that harbors the drug (blue spheres). (E) In the SMM/CK-571 structure, the converter is in a down position and is rotated by 50° with respect to the position it occupies in the PPS state (PDB ID code 1BR1, magenta). To compare the three structures, the proteins were aligned in C, D, and E using the N-ter domain as a reference.