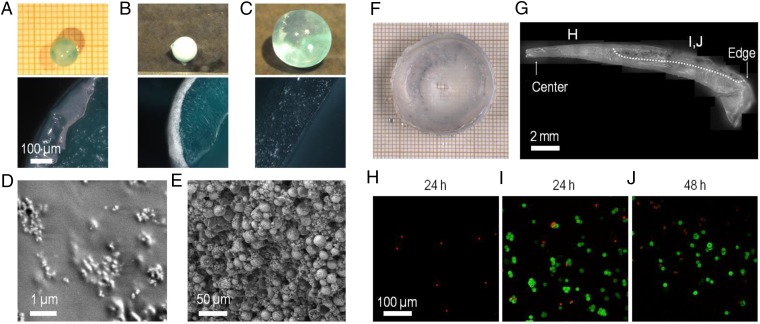
Fig. 6.
Fabrication of composite ceramic-hydrogel coatings and cell encapsulation. (A–E) Fabrication of composite hydroxyapatite–PVA hydrogel coatings. (A and B) Coating by immersion of dry PAAc–PAAm spheres in 10 wt% PVA solutions containing dispersions of hydroxyapatite nanoparticles (5 wt%) (A) or microparticles (20 wt%) (B, Top) Pictures of spheres after immersion (30 min for nanoparticles, 60 min for microparticles) and rinsing in water (15 min). (Bottom) Cross-section images by optical microscopy. (C) Same observations as B showing the absence of coating when the PAAc–PAAm sphere is swollen to equilibrium in pure water before immersion in the PVA solution with hydroxyapatite microparticles. (D and E) Low-vacuum SEM observations of the surface of the spheres coated with nanoparticles (D) and with microparticles (E). (F–J) Encapsulation of mouse fibroblasts NIH 3T3 in PVA hydrogel films using swelling of dry PEG films. (F) Cell-laden PVA hydrogel film obtained after the encapsulation protocol. (G) Cross-section of the PVA hydrogel film showing the gradient in film thickness. The picture is constructed by juxtaposing several images. The bottom surface was in contact with the PEG substrate, and the top surface with the cell suspension. Dotted line delimitates the upper region where clusters of living cells are revealed by MTT staining. (H–J) Confocal microscopy observations near the top surface of the hydrogel films for different film thicknesses: less than 1-mm thickness after 24 h (H); more than 1-mm thickness after 24 h (I) and 48 h (J). Green staining by calcein reveals the cytoplasm of living cells. Red staining by ethidium reveals the nucleus of dead cells.