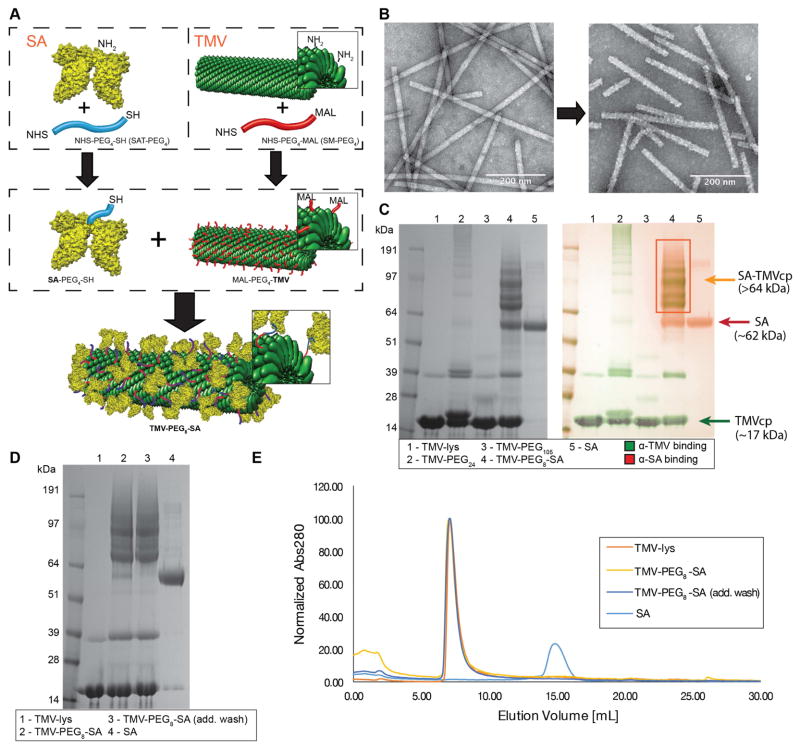
Figure 1. Coating TMV surface with SA.
A, schematic representation of the SA conjugation to TMV. Step one: Conjugation of SAT(PEG)4 and SM(PEG)4 to the lysine residues of SA protein and TMV-lys respectively (-NHS ester to -NH2 reaction). Step two: conjugation of SA-PEG4-SH to TMV-PEG4-maleimide (-SH to -maleimide reaction). Human serum albumin (HSA) was used to prepare the TMV-PEG8-SA particles. B, TEM images of VNPs before (TMV-lys, left) and after SA conjugation (TMV-PEG8-SA, right). The morphology of TMV-lys surface changes from smooth to ‘patchy’ upon SA conjugation. C, SDS-PAGE and Western Blot (WB) analysis of TMV-lys particles before and after PEGylation and SA conjugation. Free SA was used as reference. Successful conjugation of PEG is indicated by presence of bands with apparent molecular weights (>17 kDa) higher in respect to TMVcp band (~17 kDa). SA conjugation was also indicated by presence of multiple protein bands corresponding to SA-TMVcp (multiple bands of apparent Mw > 64kDa; theoretical molecular weight of 1:1 SA:TMVcp monomer = 83 kDa;) as shown by WB immune recognition. Additional WB images are shown Supplementary Figure S2. D, SDS-PAGE analysis of TMV-PEG8-SA before and after additional wash in PBS. Reduction of non-coupled SA band (apparent Mw of ~62 kDa) can be observed. E, Size exclusion chromatography (SEC) analysis of TMV-PEG8-SA before and after additional washes. 100 μg of TMV conjugates were analyzed. No significant amount of free SA has been detected thus the non-coupled SA (visible in panels C + D) is expected to be physically adsorbed on the surface of TMV-PEG8-SA particles. SA was used as reference.