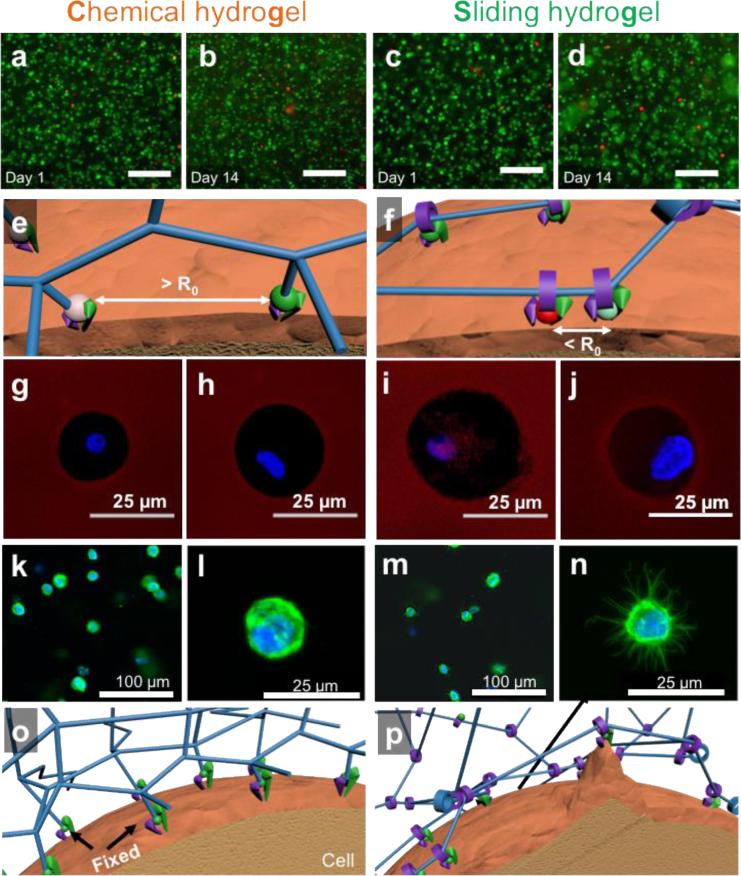
Figure 3. Sliding hydrogels, but not chemical hydrogels, facilitate cells to reorganize ligands and cytoskeleton in in 3D.
(a-d) Fluorescent microscopy images of encapsulated human mesenchymal stem cells 1 day and 14 days after encapsulation in chemical hydrogel or sliding hydrogel stained with calcium-AM (green; highlights live cells) and ethidium homodimer (red; highlights dead cells). The scale bar is 100 μm in panels (a) to (d). (e) Fluorescein isothiocyanate- (green ball) and tetramethylrhodamine- (red ball) labeled ligands are immobilized in chemical hydrogels with a distance greater than the Förster distance (R0). (f) Fluorescein isothiocyanate and tetramethylrhodamine-labeled ligands in sliding hydrogels cluster together by sliding along the hydrogel network, resulting in a distance less than R0. (g-j) Assessment of the ability of cells to reorganize ligands in the hydrogels via fluorescence resonance energy transfer, by taking confocal microscopy images of tetramethylrhodamine emission from chemical hydrogels (g) or sliding hydrogels (i) with cells encapsulated excited with a 488-nm laser. Cells treated with blebbistatin to inhibit myosin contraction were used as controls in chemical hydrogels (h) and sliding hydrogels (j). FRET signals were only observed in sliding hydrogels, suggesting molecular ligand mobility in sliding hydrogels but not in chemical hydrogels. Blebbistatin treatment abolished the ability of cells to pull on mobile ligands, with no changes in FRET signals (h, j). (k-n) Assessment of the ability of cells to change morphology in hydrogels, by taking confocal microscopy images of cells in chemical hydrogels (k-l) and sliding hydrogels (m-n) stained for F-actin (green) and the nucleus (blue). Higher-resolution images of cell morphology are shown in (l,n). (o,p) Schematic illustrations of the difference in molecular ligand mobility on cell morphology in hydrogels by reorganizing ligands and crosslinks in 3D.