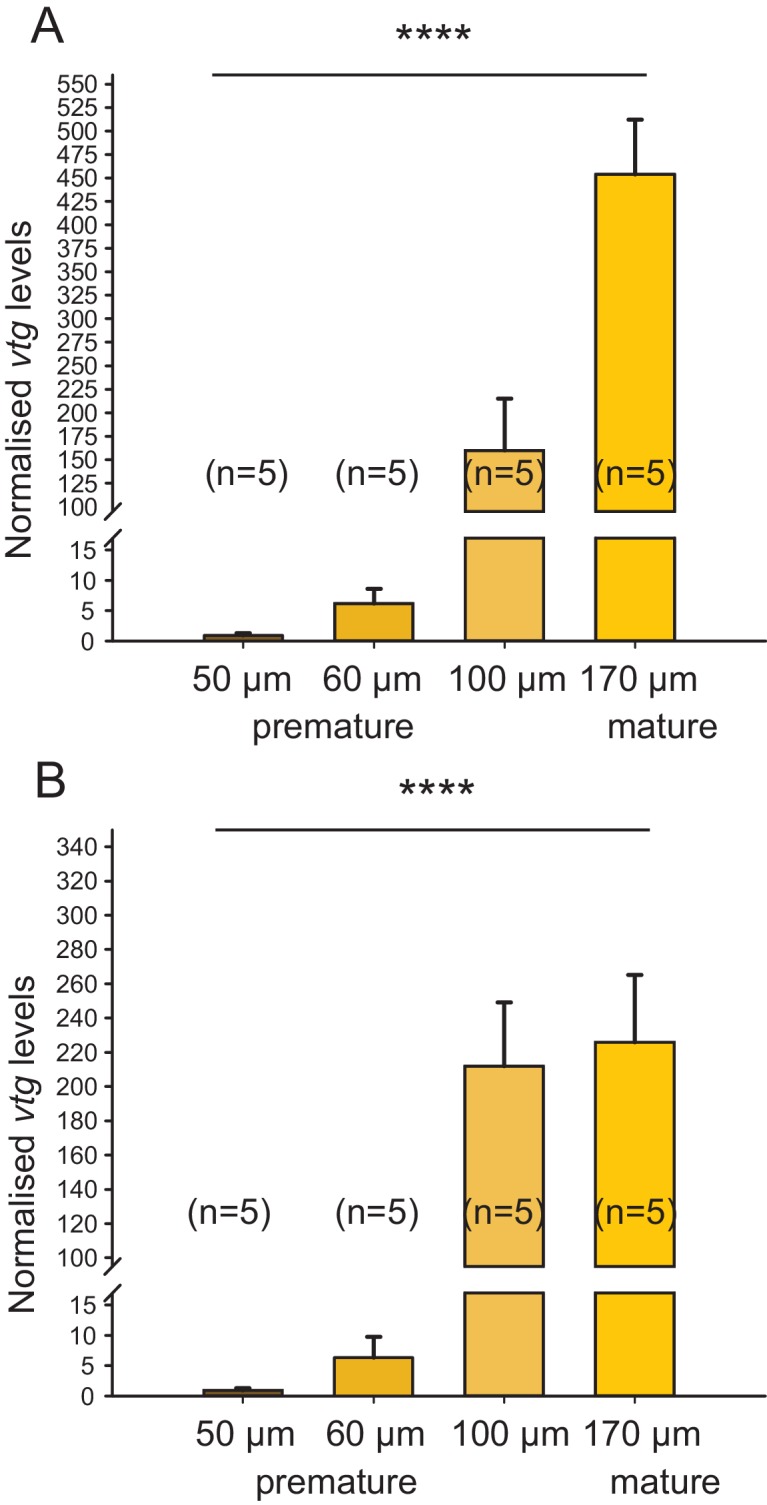
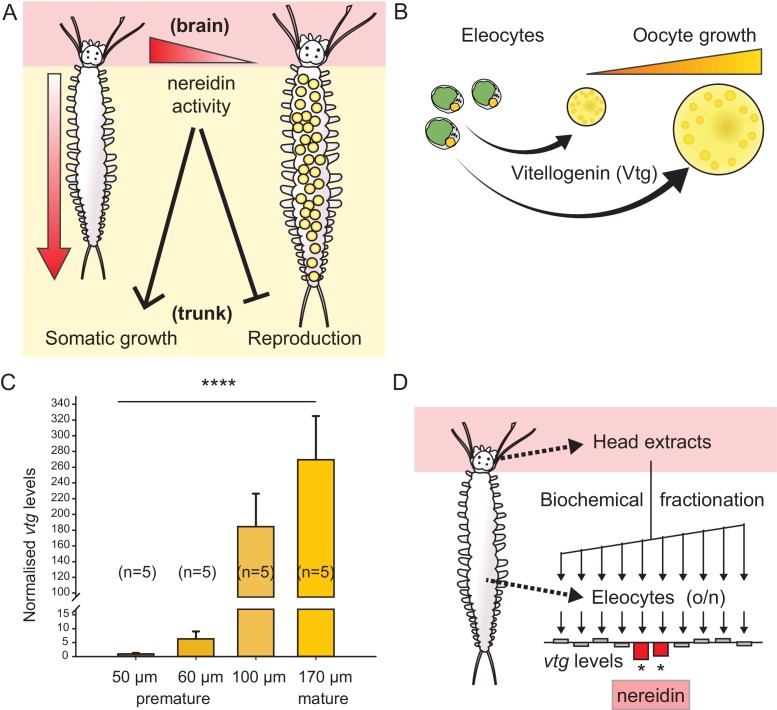
Figure 1. Vitellogenin expression in eleocytes is a quantitative measure for the maturation stage of Platynereis, allowing for the establishment of a bioassay to purify the enigmatic brain hormone, nereidin.
(A) Scheme summarising the critical role of the brain hormone nereidin in energy expenditure, as derived from classical experiments: Before maturation (left), high nereidin levels sustain somatic growth, but repress reproduction; drops in nereidin activity levels initiate the generation of germ cells and sexual maturation (right). (B) Eleocytes in the worm’s coelom have a central role in reproductive commitment, as they synthesise the yolk protein precursor Vitellogenin (Vtg); after release into the coelomic fluid, Vtg is endocytosed by the developing oocytes, leading to oocyte growth. (C) Expression levels of vtg in eleocytes are significantly up-regulated during maturation, confirming vtg as a suitable molecular marker to quantify maturation state. qRT-PCR quantification of vtg levels in eleocytes sampled at different stages of sexual maturation (as assessed by oocyte diameter). The increase in vtg transcripts from premature eleocytes (oocyte diameter: 50 and 60 µm) to those from mature eleocytes (oocytes diameter: 170 µm) is evident; expression levels were calculated against the arithmetic mean of the reference genes cdc5 and rps9, and normalised to the expression of vtg in eleocytes from animals with an oocyte diameter of 50 µm. Statistical significance was tested an one-way ANOVA. ***p<0.001. n: number of biological replicates. (D) Resulting bioassay for the purification of nereidin from head extracts: primary cell cultures were derived from coelomic cells of premature animals; vtg levels were quantified after overnight incubation with different fractions of head extract to determine those fractions containing significant inhibitory activity (nereidin). Data for panel (C) provided in Figure 1—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.17126.003
Figure 1—figure supplement 1. Identification of a Platynereis Vitellogenin orthologue.

Figure 1—figure supplement 2. Irrespective of the chosen reference gene, vitellogenin is regulated over the course of maturation.