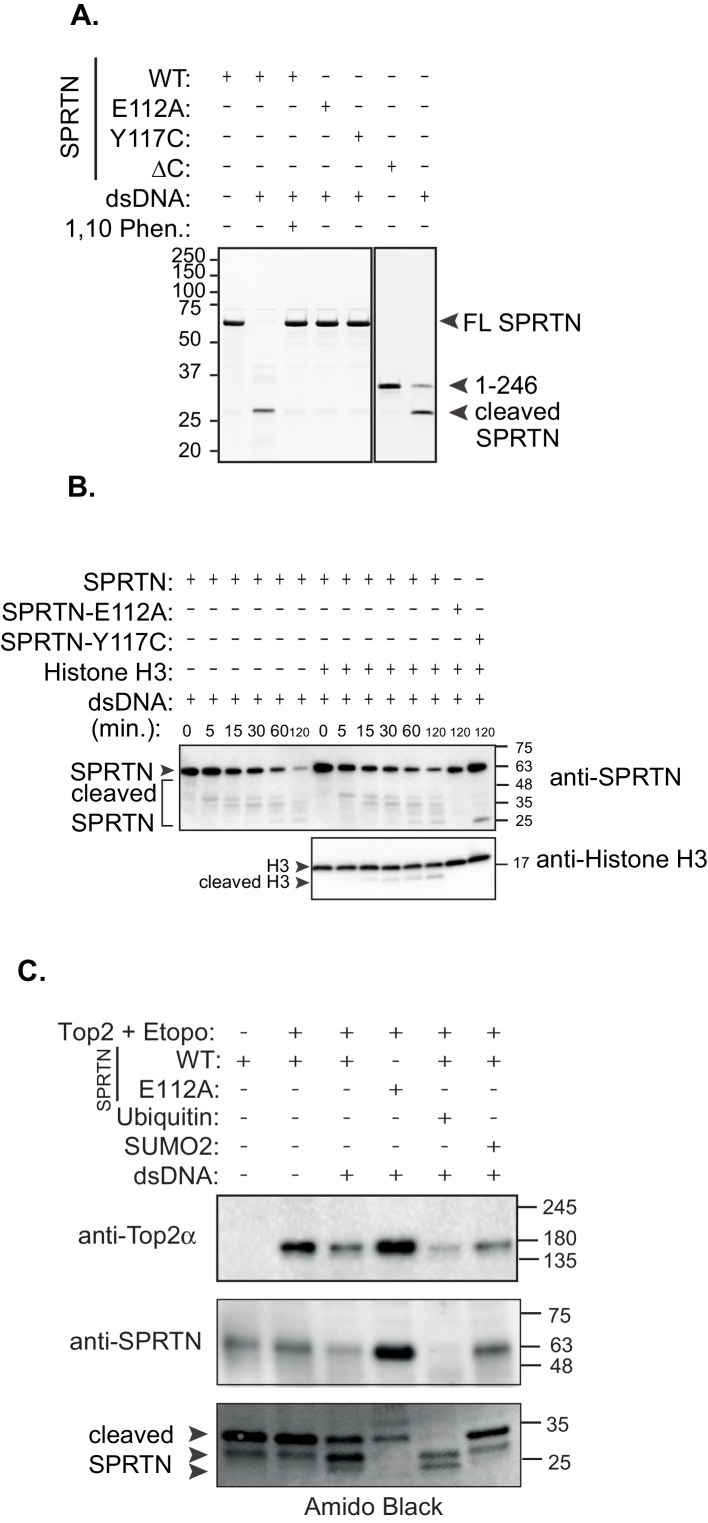
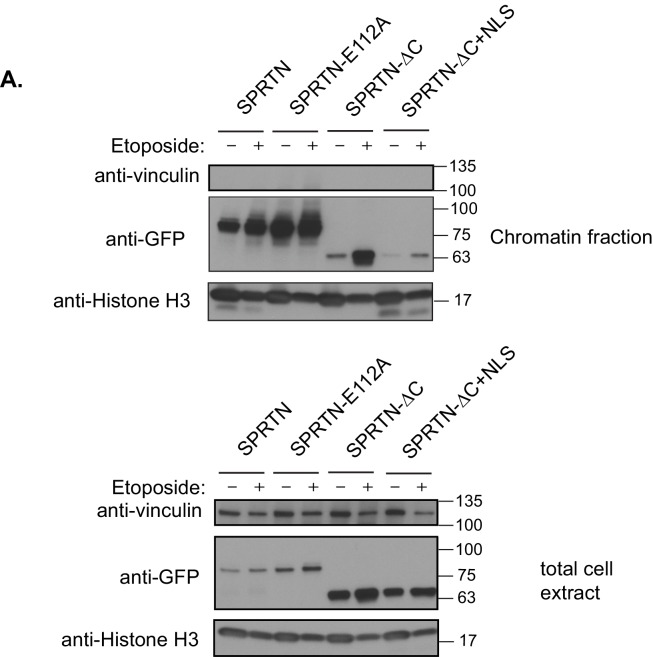
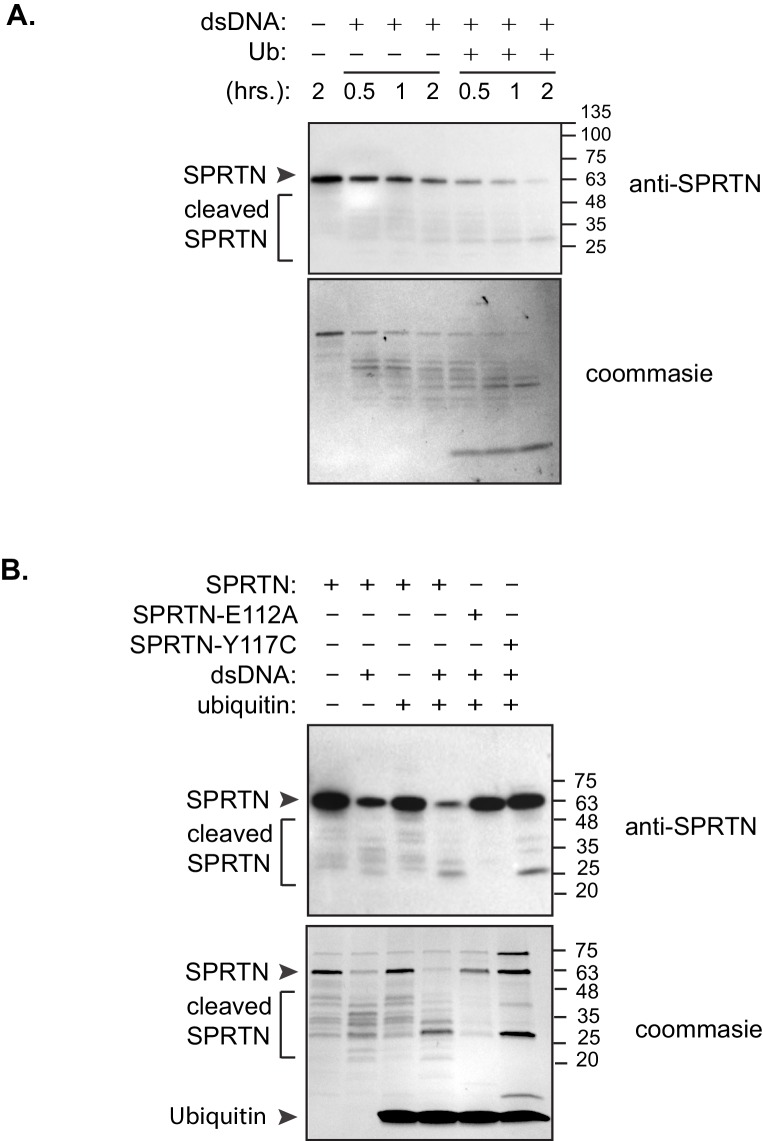
Figure 4. In vitro SPRTN substrate cleavage.
(A) In vitro SPRTN self-cleavage reactions. Purified proteins were incubated with or without DNA for 5 hr at 37°C. 1,10 Phe = 1,10 Phenanthroline, a zinc metal chelator and inhibitor of zinc metalloproteases. Proteins were separated by SDS-PAGE and stained with coommasie blue. (B) In vitro histone H3 cleavage. SPRTN was incubated with or without histone H3 (SPRTN:H3 molar ratio of 4:1) in the presence of dsDNA for the indicated time points. SPRTN-E112A or SPRTN-Y117C mutants were incubated for 2 hr. Proteins were separated on an SDS-PAGE gel and transferred to a membrane for Western blot analysis. Histone H3 cleavage as well as SPRTN self-cleavage were monitored by immunobloting with antibodies against histone H3 and SPRTN. (C) In vitro Top2 cleavage. Purified recombinant Top2 was pre-incubated with DNA and Etoposide to irreversibly bind Top2 to DNA. Recombinant SPRTN or SPRTN-E112A was then added alone or in combination with 10-fold molar excess of either ubiquitin or SUMO and incubated for 2 hr at 37°C. Proteins were separated by SDS-PAGE and transferred to a membrane for Western blot analysis. Membrane was stained with amido black to detect SPRTN cleavage fragments.