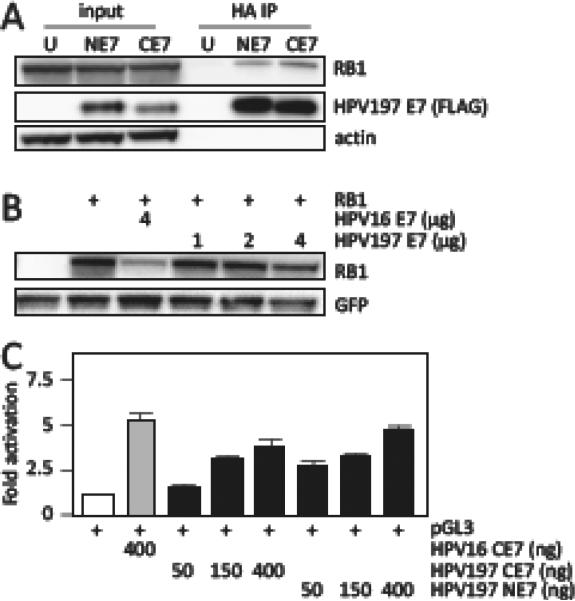
Figure 3.
Binding of HPV197 E7 to the RB1 tumor suppressor. (A) Immunoprecipitation/western blot experiments documenting interaction of ectopically expressed amino (NE7) and carboxyl (CE7) terminally HA/FLAG epitope tagged HPV197 E7 proteins with endogenously expressed RB1 in HCT116 cells. Untransfected (U) cells were used as controls. Input lanes contain 3.3 % of protein extracts used for HA immunoprecipitation (HA IP). HPV197 E7 levels were assessed by immunoblotting with a FLAG antibody. (B) RB1 destabilization assays performed by transient expression of the indicated proteins in Saos-2 osteosarcoma cells. RB1 steady state levels were determined by western blotting. A GFP blot is shown to normalize RB1 levels by transfection efficiency. HPV16 E7 was used as a positive control for RB1 destabilization. (C) E2F dependent transcriptional reporter assays. The corresponding plasmids were transfected into U-2 OS osteosarcoma cells and firefly luciferase activity was determined. Values were normalized to co-transfected renilla luciferase expression and represent averages and standard deviations from an experiment performed in triplicate. HPV16 E7 was used as a positive control for induction of E2F transcriptional activity. Similar results were obtained in three independent experiments.