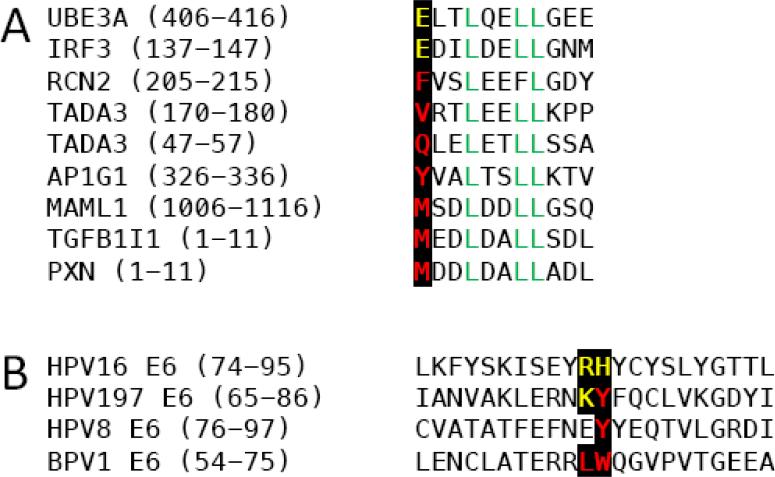
Figure 4.
(A) Sequence alignments of LXXLL motifs contained in cellular proteins associated with HPV197 E6. Negatively charged and hydrophobic amino acid residues at the −3 position and the LXXLL sequence are highlighted. (B) Alignment of sequences corresponding to the alpha helix 2 and beta sheet 4 of the BPV1 and HPV16 E6 sequences and the corresponding HPV8 and HPV197 E6 sequences. Basic residues shown to be critical for binding of LXXLL proteins with an acidic residue at −3 and hydrophobic residues critical for LXXLL protein binding with hydrophobic amino acid residues at −3 are highlighted.