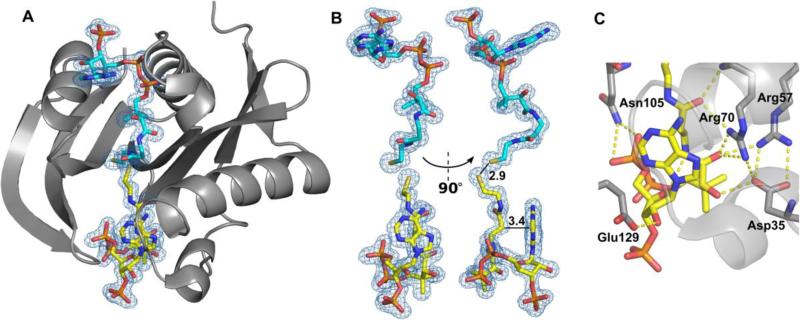
Figure 3. Structure of SACOL1063 in complex with CoA.
(A) Two CoA molecules were bound - one in the canonical AcCoA/CoA-binding site (cyan), and one in the acceptor-substrate-binding site (yellow). 2Fo-Fc electron density map of the bound CoA molecules is presented (σ=1.0). (B) The CoA molecule bound in the acceptor-substrate-binding site (yellow) is very sharply bent. The distance of 2.9 Å between the two sulphur atoms of the two CoA molecules, and the closest distance (3.4 Å) between the pantothenate and adenine groups of the CoA molecule bound in the acceptor-substrate-binding site are indicated. (C) Residues involved in binding of CoA in the acceptor-substrate-binding site.