Abstract
Purpose
This study was designed to investigate the effects of total parenteral nutrition (PN) using different lipid emulsions in patients undergoing major abdominal surgery.
Methods
Fifty-two patients were randomized to receive soybean oil + medium chain triglycerides (MCT) (group I), soybean oil + olive oil (group II), soybean oil + olive oil + fish oil (group III) as a lipid source. PN was started on postoperative day 1 and patients were maintained on PN for a minimum period of 4 days. Laboratory variables (CRP, prealbumin, transferrin) were measured before surgery and on postoperative days.
Results
Three treatment groups were included in the study. Patients in group I received long chain triglycerides (LCT) + LCT/MCT emulsion (%75 LCT + %25 LCT/MCT); Patients in group II received olive oil based emulsion (80% olive oil + 20% soybean oil, ClinOleic); Patients in group III received fish oil in addition to olive oil based emulsion (%85 ClinOleic + %15 Omegaven; Fresenius Kabi). The following 14 parameters were assessed: body weight, CRP, prealbumin, transferrin, tumor necrosis factor-α, interleukin-6, total antioxidant status, thiobarbituric acid reactive substances, oxidized low density lipoprotein-2, complete blood cell, international normalized ratio, D-dimer, activated partially thromboplastin time, prothrombin time. All other parameters showed no differences among the groups.
Conclusion
The results of our trial demonstrate a potential beneficial effect of soybean oil/olive oil based lipid emulsions for use in PN regarding inflammatory response and oxidant capacity in the treatment of patients.
Keywords: Lipid emulsions, Inflammatory response, Total antioxidant status
INTRODUCTION
Nutritional intervention is an essential part of postoperative care. Parenteral nutrition (PN) is commonly administrated after major abdominal surgical procedures due to the limitations of enteral nutrition at early postoperative or posttraumatic stages [1]. Artificial nutrition in surgical patients evolved dramatically from a supportive therapy to a therapeutic role. Recent advances have shown that supply of selective additives to nutritional regimens can influence inflammatory and immunological processes [1]. Among various nutrients lipids have attracted special attention [2]. Lipid emulsions are commonly included in PN regimens both as part of energy supply and as a source of essential fatty acids. However, evidence suggests that besides their nutritional role, lipids have other biological functions via the alteration of the fatty acid composition of cell membranes [3]. It has been shown that the fatty acid composition of cell membranes is strongly influenced by the fatty acid profile of dietary lipids [4]. Experimental and clinical trials have shown that lipids are capable of modulating immune response through their effect on lipid-mediator synthesis, cytokine release, leukocyte activity, and endothelial cell activation [5].
Conventional lipid emulsions are based on soybean and/or safflower oil. They are very rich in ω-6 polyunsaturated fatty acids (PUFA). Evidence suggests that an excessive intake of ω-6 PUFA in PN is associated with an unbalanced fatty acid pattern in cell membranes which may lead to a modification of the production of lipid mediators, namely prostoglandins and leukotrienes, and a promotion of immunosupression and systemic inflammatory reactions [6]. Increased oxidative stress from high dose of ω-6 PUFA delivered to the patient is another suspected adverse effect of these emulsions [4]. Recently, preparations containing a mixture of long chain triglycerides (LCT) together with medium chain triglycerides (MCT) have been used in clinical practice to take the advantages of the properties of these 2 lipids. MCT/LCT lipid emulsions have a higher degree of immediate oxidation and a positive influence on the immune system due to the reduced amount of ω-6 PUFA [7]. More recently, research on the importance of fatty acids for inflammatory response led to research for new types of lipid emulsions and olive oil or fish oil based lipid emulsions have been introduced. Olive oil based lipid emulsions with a high content of monounsaturated fatty acids (MUFA) have been reported to reduce lipid peroxidation and to have neutral effects on the immune response [8]. Fish oil-containing solutions are considered to have antiinflammatory properties due to their high content of ω-3 PUFA. Omega-3 PUFA are capable of modulating lipid-mediator synthesis, cytokine release, leukocyte activity, and endothelial cell activation [9].
The present study was designed to investigate the effects of total PN containing three different lipid emulsions in patients undergoing major abdominal surgery.
METHODS
The study protocol was reviewed and approved by our Institutional Ethical Committee. The nature and purpose of the study were explained to the subjects and a written informed consent form was obtained before inclusion into the study. The procedures followed were in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Cancer patients planned to undergo elective major gastrointestinal tract surgery were enrolled in the study. Exclusion criteria were: (1) preoperative enteral or PN support; (2) organ failures (renal, respiratory, heart, liver); (3) diabetes mellitus type 1 or 2; (4) ongoing infection; (5) biological evidence of inflammation and/or sepsis; (6) current treatment with corticosteroids, nonsteroid anti-inflammatory or immune-suppressive drugs; (7) contraindications for PN (any state of shock, serum lactate> 3–4 mmol/L, hypoxia (pO2 < 50 mmHg), severe acidosis (pH < 7.2, pCO2 > 80 mmHg).
Patients were randomly assigned in 3 groups according to the lipid source of PN. Group I (n = 18) received LCT + LCT/MCT emulsion (%75 LCT + %25 LCT/MCT, Intralipid; Baxter, Deerfield, IL, USA or Lipovenoes; Fresenius + Lipofundin; Braun, Kronberg im Taunus, Germany). We used a mixture of LCT and LCT/MCT with a ratio of 75% LCT + 25% LCT/MCT. Previous data have demonstrated that when mixed with parenteral nutrients as an all-in-one admixture, lipid emulsions composed of MCTs and LCTs yield more stable formulations compared with those compounded with pure LCT lipid emulsions [8]. The ideal combination of MCT/LCT has been demonstrated to be 25%/75% by previous studies. There is no commercially available intravenous lipid emulsion containing this combination of triglycerides. Therefore, we prepared it by admixing LCT/MCT to pure LCT. Group II (n = 13) received olive oil based emulsion (80% olive oil + 20% soybean oil, ClinOleic; Baxter) and group III (n = 21) received fish oil in addition to olive oil based emulsion (%85 ClinOleic + %15 Omegaven; Fresenius Kabi, Bad Homburg, Germany). The basal PN solutions were isonitrogenous and identical in nutrient composition except for differences in lipid source. PN was started on postoperative day 1 and patients were maintained on PN for a minimum period of 4 days.
Blood samples were drawn and laboratory variables (CRP, prealbumin, transferrin, tumor necrosis factor [TNF]-α, interleukin (IL)-6, total antioxidant status [TAS], thiobarbituric acid reactive substances [TBARS], oxidized low density lipoprotein-2 (oxLDL-2), common blood count [CBC], international normalized ratio [INR], D-dimer, activated partial thromboplastin time, prothrombin time) were measured before surgery and on post-operative day 1 before first dosing (baseline) and day 4.
Plasma TAS was assessed by the TAS which measures the capacity of plasma to quench the stable free radical species generated from 2,20 azinobis-(3 ethylbenzothiazoline-6-sulphonic acid (ABTS), (Randox Laboratories, Crumlin, UK) [4]. TBARS, as an index of lipid peroxidation, hence oxidative stress, were measured by previously described method.
Statistical analysis
The safety analyses were based on the safety population that was defined as all patients who received at least one dose of the study medication. The efficacy analyses were based on the intent-to-treat population that was defined as all patients in the safety population who provided baseline and post baseline assessments for at least one efficacy parameter.
The following 14 laboratory parameters were assessed: body weight, CRP, prealbumin, transferrin, TNF-α, IL-6, TAS, TBARS, oxLDL-2, CBC, INR, D-dimer, activated partial thromboplastin time, prothrombin time. Changes from the baseline in the above efficacy assements were analyzed using an analysis of variance model with the treatment effects, where the baseline was the last assessment before the patient received study medication. All null hypotheses of no treatment differences were tested at the two-sided, 5% level. A P-value less than 0.05 was considered significant. Data were presented as mean ± standard deviation.
RESULTS
A total of 52 patients, 19 women and 33 men, were enrolled in this study. There were no statistically significant differences between the 3 groups regarding age, sex, SGA scores, body mass indexes, and nutrition risk indexes of the patients (P > 0.05) (Tables 1, 2).
Table 1. Patient characteristics.
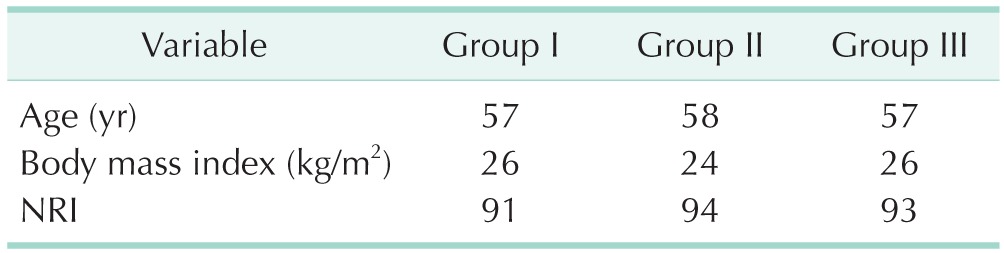
Group I. soybean oil + medium chain triglycerides; group II, soybean oil + olive oil; group III, soybean oil + olive oil + fish oil; NRI, nutriutonal risk ındex.
Table 2. Subjective global assessment (SGA) scores of the patients in 3 study groups.
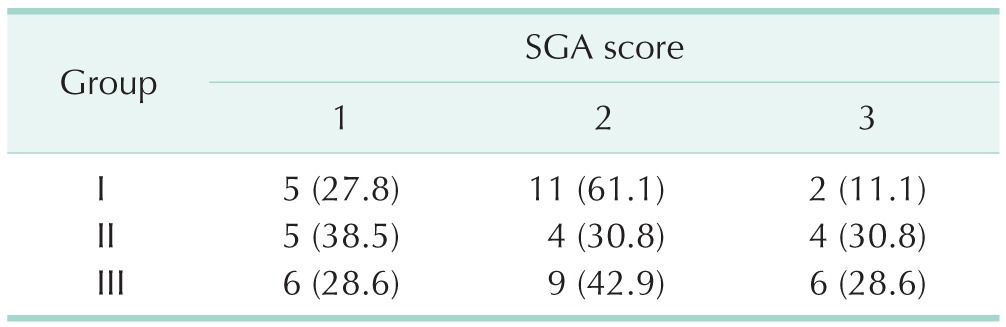
Values are presented as number (%).
Group I. soybean oil + medium chain triglycerides; group II, soybean oil + olive oil; group III, soybean oil + olive oil + fish oil.
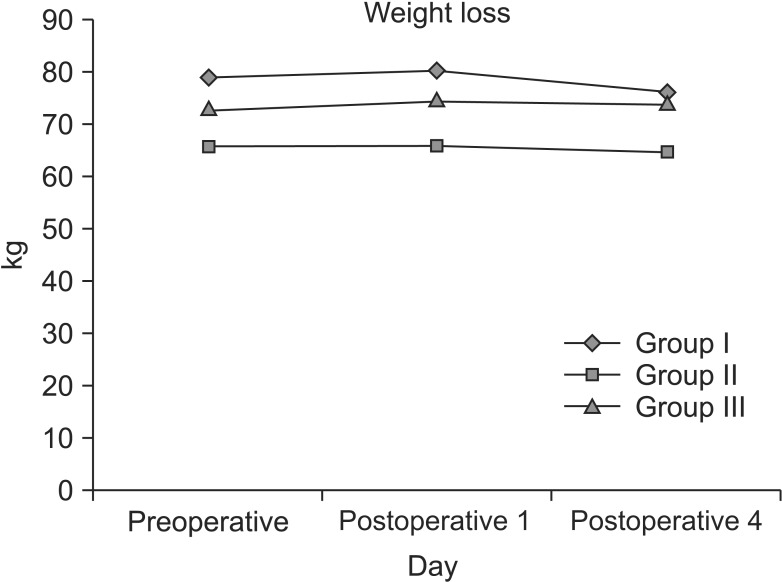
On the average, patients in group I (75% LCT/25% LCT/MCT) lost 2.4 kg of weight by day 4; patients in groups II (80% olıve oıl + 20% soybean oıl) and III (85% clınoleıc + 15% omegaven) gained 0.2 kg and 0.3 kg by day 4, respectively (Fig. 1). There were no treatment differences between groups II and III (P = 0.9913). Groups II and III were statistically significantly higher than group I (P < 0.0308).
Fig. 1. Evaluation of body weight from preoperative body weight and on postoperative day 1 and 3 during the study period. There was no statistically significant difference between the groups. Group I, soybean oil + medium chain triglycerides; group II, soybean oil + olive oil; group III, soybean oil + olive oil + fish oil.
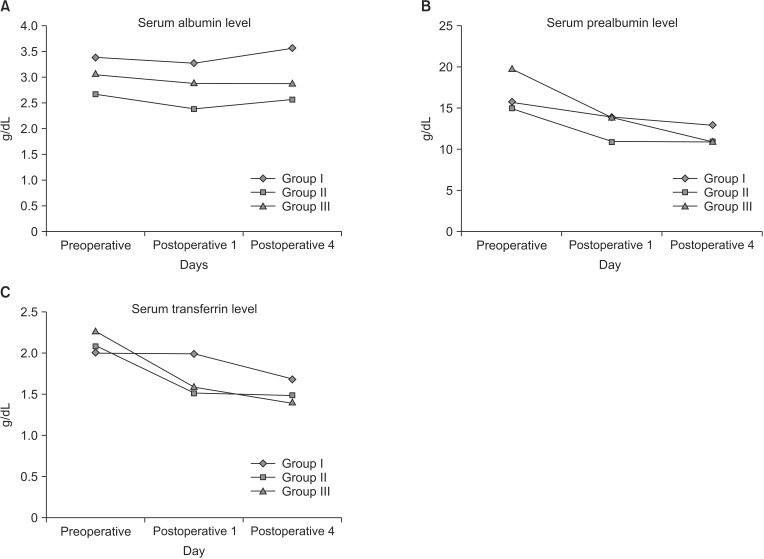
A decrease from baseline in serum prealbumin levels was observed in all groups on day 4. The means of the reduction were 0.8, 0.1, and 2.4 for groups I, II, and III, respectively (Fig. 2A). None of the paired treatment comparisons were statistically significant (P ≥ 0.5373). A decrease from baseline in serum transferrin levels was observed in all groups on day 4. The means of the reduction were 0.2, 0, and 0.2 for groups I, II, and III, respectively (Fig. 2B). None of the paired treatment comparisons were statistically significant (P ≥ 0.2151). An increase from baseline in serum albumin levels was observed in all groups on day 4. The means of the increase were 0.5, 0.4, and 0.3 for groups I, II, and III, respectively (Fig. 2C). None of the paired treatment comparisons were statistically significant (P ≥ 0.2753).
Fig. 2. Assessment of nutritional parameters, serum albumin (A), prealbumin (B), and transferrin (C) levels, before and after the operation. Group I, soybean oil + medium chain triglycerides; group II, soybean oil + olive oil; group III, soybean oil + olive oil + fish oil.
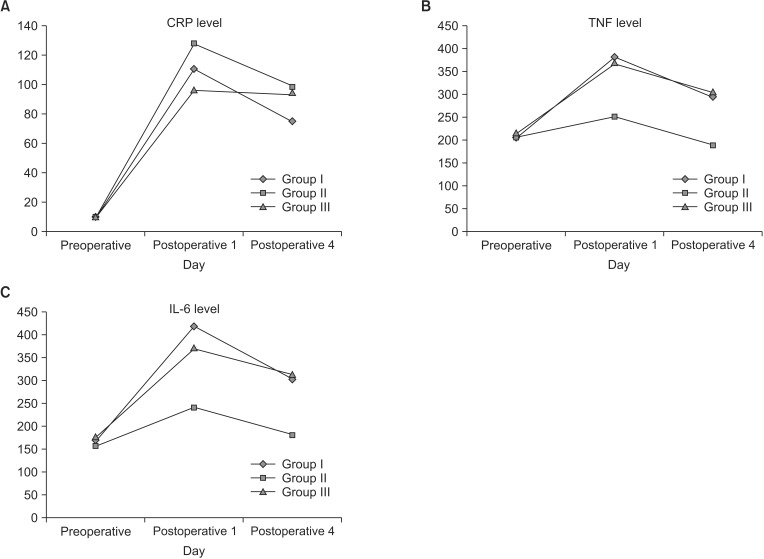
The means of the changes from baseline in CRP levels were –45.6, –34.7, and 1.5 for groups I, II, and III, respectively (Fig. 3A). None of the paired treatment comparisons were statistically significant (P ≥ 0.3733). The means of the changes from baseline in TNF-α levels (Fig. 3B) were –92.2, –60.6, and –67.5 for groups I, II, and III, respectively. None of the paired treatment comparisons were statistically significant (P ≥ 0.2789). The means of the changes from baseline in IL-6 levels (Fig. 3C) were –109, –57.7, and –62.9 for groups I, II, and III, respectively. None of the paired treatment comparisons were statistically significant (P ≥ 0.0710).
Fig. 3. Assessment of inflammatory and immunological parameters, CRP (A), tumor necrosis factor (TNF)-α (B), and interleukin (IL)-6 (C) levels, before and after the operation. Group I, soybean oil + medium chain triglycerides; group II, soybean oil + olive oil; group III, soybean oil + olive oil + fish oil.
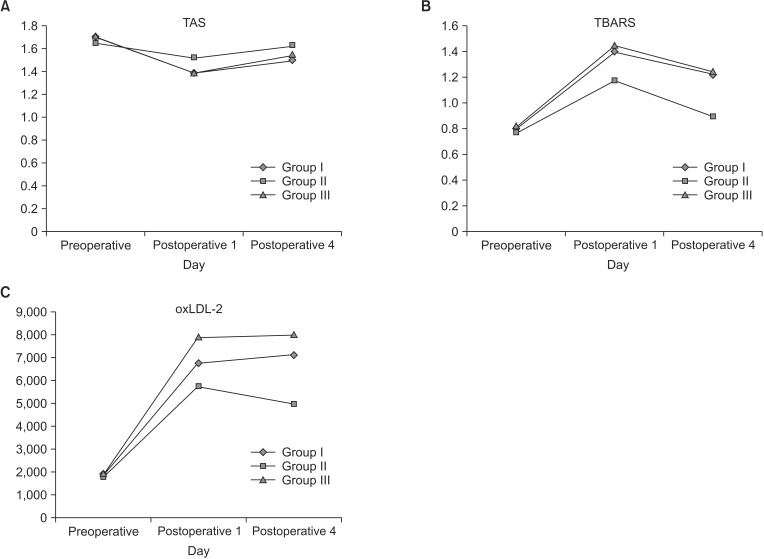
The means of the changes from baseline in TAS levels were approximately 0.1 for the three treatment groups (Fig. 4A). None of the paired treatment comparisons were statistically significant (P ≥ 0.0864). Group II demonstrated approximately double the amount of reduction in TBARS that groups I and III had by day 4 (Fig. 4B). The differences were statistically significantly (P ≤ 0.0015) even after making adjustments for multiple comparisons via the Bonferroni procedure. There were no treatment differences between groups I and III. The means of the changes from baseline in oxLDL3 levels (Fig. 4C) were 343.2, –444, and 225.0 for groups I, II, and III, respectively. None of the paired treatment comparisons were statistically significant (P ≥ 0.0688). Similarly, no treatment differences were observed for oxLDL-1 and -2 levels.
Fig. 4. Assessment of total antioxidant status (TAS; A), thiobarbituric acide reactive substance (TBARS; B), and oxidized low density lipoprotein-2 (oxLDL-2; C) before and after the operation. Group I, soybean oil + medium chain triglycerides; group II, soybean oil + olive oil; group III, soybean oil + olive oil + fish oil.
The coagulation parameters, platelet counts, leukocyte counts, and D-dimer levels were not different in the 3 groups.
DISCUSSION
Nutritional modulation of immune function has become an important issue in clinical practice. It has been widely accepted that lipids play an important role in pharmaconutritional regulation of the immune function and inflammatory response thereby influencing patient outcomes [2]. Surgical trauma induces a general inflammatory response [10]. Systemic proinflammatory mediators increase early, followed by an elevation in systemic IL-6 that triggers the initiation of acute phase response. Some patients show an early hyperinflammation that might be damaging to the host. The hyperinflammatory response is characterized by overproduction of TNF-α, IL-1β IL-6, and IL-8. In this situation, anti-inflammatory effects of the fatty acids in PN may provide a benefit. A compensatory anti-inflammatory response syndrome follows the acute phase response and more immunoneutral lipids may be beneficial in this second phase.
Recently, the focus has been oriented towards olive oil and fish oil based lipids for use in PN. Numerous experimental and clinical studies have demonstrated the immune modulating properties of fish oil [8]. Fish oil markedly suppresses monocyte generation of pro-inflmammatory cytokines and promotes leukotriene and tromboxane production of less biological activity [4]. The ability of fish oil to decrease the production of inflammatory response cytokines and eicosanoids was demonstrated in various patient groups and suggested that fish oil might be a useful agent in aid of the control of systemic inflammatory response. There are a number of studies that have investigated the effects of fish oil on postsurgical patients. A randomized controlled study Wachtler at al. [11] demonstrated that systemic levels of IL-10, IL-6, and TNF-α were decreased in surgical patients after administration of fish oil supplemented PN. Weiss et al. [12] demonstrated a significant down regulation of the proinflammatory response in patients after abdominal surgery if these patients were treated with fish oil based lipid emulsion during the perioperative period.
At the same time, an increased production of markers like IL-2 and IFN-γ shows that the administration of 0.2 g/kg per day of fish oil is not immunosuppressive [13]. A significantly shorter hospital stay after major abdominal surgery was reported in patients receiving postoperative [14] and perioperative [15] fish oil supplements. In a recent published study, the effect of postoperative supplementation of parenteral ω-3 PUFA was evaluated in colorectal cancer patients [16]. Lower levels of IL-6 and TNF-α were observed in the fish oil group and it was concluded that fish oil may modulate immune response. Taken together, these data suggest that PN enrichment with fish oil may be able to prevent hyperinflammatory situations after major surgery.
Olive oil is popular for its antioxidant properties, especially regarding cardiovascular pathologies [17]. Less attention has been paid to the effects of olive oil on the immune system. Results of animal studies suggest that olive oil offers an immunologically neutral alternative to conventional lipid emulsions for use in PN, with the potential benefit of some mild anti-inflammatory effects [18]. The precise mechanism of this anti-inflammatory effect is unclear [8]. MUFA may have an anti-inflammatory effect by diluting the ω-6 PUFA in the emulsion and competing with it for insertion into the phospholipids of cell membranes.
In the present study, we aimed to evaluate the effect of three lipid emulsions with different fatty acid compositions on inflammatory reaction and antioxidant status in patients undergoing major abdominal surgery. We compared the effects of a conventional LCT/MCT emulsion, an olive oil based emulsion and fish oil supplemented olive oil based emulsion. The postoperative increases of circulating CRP and TNF-α concentration did not differ between the study groups but after 4 days of PN, plasma TNF-α and IL-6 concentrations were lower in patients receiving the olive oil based lipid emulsions.
It has been found that the effects of PUFA on immune and inflammatory responses do not solely depend on eicosanoid generation [4]. Lipid emulsions for PN also influence the antioxidant status of the patient. PUFA in the presence of oxygen can be peroxidated and degraded into radicals, resulting in oxidative stress and toxicity [19]. A number of genes involved in the inflammatory response, including those encoding for TNF-α, IL-1, IL-6, and platalet derived growth factor, are modulated by exposure to oxidized LDL. A low level of fatty acid unsaturation will decrease oxidative stress. The indirect actions of ω-9 MUFA, as anti-inflammatory agents, may be attributable to reduction in the level of oxidant stress. MUFA is less prone to peroxidation than PUFA [8]. Polyphenols in the olive oil may also exert indirect anti-inflammatory effects by virtue of their antioxidant properties [20]. We observed that the lowest concentrations of oxLDL3 were in group II. Measurement of TBARS concentration, although nonspecific, is widely used as an indicator of lipid peroxidation process and, indirectly, of oxidative stress. In the present study, lipid peroxidation measured as TBARS levels were lowest in patients receiving olive oil based lipid emulsion. Also, the decrease in TAS levels, representing antioxidant status was lower in the olive oil based lipid emulsion group compared to the other 2 groups. These results suggest a better antioxidant status after major abdominal surgery with the olive oil based lipid emulsion. We may conclude that olive oil improves antioxidant status in patients undergoing major surgery.
There is an ongoing debate about the ideal composition of lipid emulsions in PN. According to current recommendations, lipid emulsions should be composed of a reduced content of LCT, counterbalanced by MCT, and long chain ω-3 PUFA. Our data show that clinical trials should also focus on the use of olive oil after major surgery. Larger prospective, randomized, double-blind trials with comparable PN regimens are required to evaluate the impact of olive oil and fish oil on clinical outcome.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Calder PC. Immunonutrition in surgical and critically ill patients. Br J Nutr. 2007;98(Suppl 1):S133–S139. doi: 10.1017/S0007114507832909. [DOI] [PubMed] [Google Scholar]
- 2.Wirtitsch M, Wessner B, Spittler A, Roth E, Volk T, Bachmann L, et al. Effect of different lipid emulsions on the immunological function in humans: a systematic review with meta-analysis. Clin Nutr. 2007;26:302–313. doi: 10.1016/j.clnu.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Berdanier CD. Role of membrane lipids in metabolic regulation. Nutr Rev. 1988;46:145–149. doi: 10.1111/j.1753-4887.1988.tb05408.x. [DOI] [PubMed] [Google Scholar]
- 4.Mantzioris E, James MJ, Gibson RA, Cleland LG. Differences exist in the relationships between dietary linoleic and alpha-linolenic acids and their respective long-chain metabolites. Am J Clin Nutr. 1995;61:320–324. doi: 10.1093/ajcn/61.2.320. [DOI] [PubMed] [Google Scholar]
- 5.Calder PC. Dietary fatty acids and the immune system. Nutr Rev. 1998;56(1 Pt 2):S70–S83. doi: 10.1111/j.1753-4887.1998.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 6.Pomposelli JJ, Bistrian BR. Is total parenteral nutrition immunosuppressive? New Horiz. 1994;2:224–229. [PubMed] [Google Scholar]
- 7.Mascioli EA, Bistrian BR, Babayan VK, Blackburn GL. Medium chain triglycerides and structured lipids as unique non-glucose energy sources in hyperalimentation. Lipids. 1987;22:421–423. doi: 10.1007/BF02537272. [DOI] [PubMed] [Google Scholar]
- 8.Sala-Vila A, Barbosa VM, Calder PC. Olive oil in parenteral nutrition. Curr Opin Clin Nutr Metab Care. 2007;10:165–174. doi: 10.1097/MCO.0b013e32802bf787. [DOI] [PubMed] [Google Scholar]
- 9.Driscoll DF, Bacon MN, Bistrian BR. Physicochemical stability of two types of intravenous lipid emulsion as total nutrient admixtures. JPEN J Parenter Enteral Nutr. 2000;24:15–22. doi: 10.1177/014860710002400115. [DOI] [PubMed] [Google Scholar]
- 10.Faist E, Wichmann MW, Kim C. Immunosuppression and immunomodulation in surgical patients. Curr Opin Crit Care. 1997;3:293–298. [Google Scholar]
- 11.Wachtler P, Konig W, Senkal M, Kemen M, Koller M. Influence of a total parenteral nutrition enriched with omega-3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J Trauma. 1997;42:191–198. doi: 10.1097/00005373-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n-3 fatty acids. Br J Nutr. 2002;87(Suppl 1):S89–S94. doi: 10.1079/bjn2001461. [DOI] [PubMed] [Google Scholar]
- 13.Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med. 2007;35:700–706. doi: 10.1097/01.CCM.0000257465.60287.AC. [DOI] [PubMed] [Google Scholar]
- 14.Tsekos E, Reuter C, Stehle P, Boeden G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin Nutr. 2004;23:325–330. doi: 10.1016/j.clnu.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, et al. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14:2434–2439. doi: 10.3748/wjg.14.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Gutierrez V, Muriana FJ, Guerrero A, Cert AM, Villar J. Plasma lipids, erythrocyte membrane lipids and blood pressure of hypertensive women after ingestion of dietary oleic acid from two different sources. J Hypertens. 1996;14:1483–1490. doi: 10.1097/00004872-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Yaqoob P, Knapper JA, Webb DH, Williams CM, Newsholme EA, Calder PC. Effect of olive oil on immune function in middle-aged men. Am J Clin Nutr. 1998;67:129–135. doi: 10.1093/ajcn/67.1.129. [DOI] [PubMed] [Google Scholar]
- 18.Moussa M, Le Boucher J, Garcia J, Tkaczuk J, Ragab J, Dutot G, et al. In vivo effects of olive oil-based lipid emulsion on lymphocyte activation in rats. Clin Nutr. 2000;19:49–54. doi: 10.1054/clnu.1999.0076. [DOI] [PubMed] [Google Scholar]
- 19.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 20.de la Puerta R, Ruiz Gutierrez V, Hoult JR. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem Pharmacol. 1999;57:445–449. doi: 10.1016/s0006-2952(98)00320-7. [DOI] [PubMed] [Google Scholar]