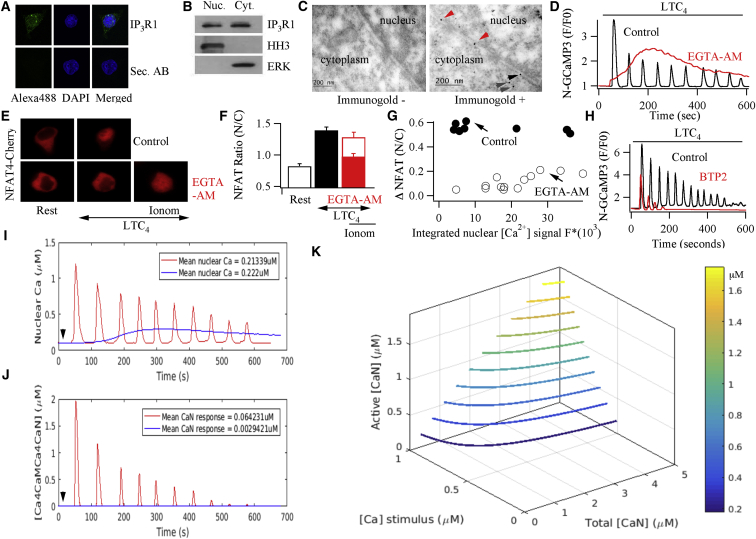
Figure 4.
Nuclear Ca2+ Oscillations Are Generated by Nuclear InsP3 Receptors and Are Effective in Maintaining NFAT4 Activity
(A) Immunostaining reveals the presence of type I InsP3 receptors in the cytoplasm and nucleus. No staining is seen when secondary antibody (Sec. AB) alone is used. Secondary antibody was conjugated with Alexa 488 for visualization.
(B) Western blots of cytoplasmic and nuclear extract show the presence of type I InsP3 receptors in both compartments. Similar results were obtained in two independent experiments.
(C) Immunogold labeling reveals the presence of type I InsP3 receptors in the inner nuclear membrane. Left-hand panel denotes a control micrograph and the right-hand one after immunogold labeling of type I InsP3 receptors. Red arrows denote InsP3R on the inner nuclear membrane, black arrow shows receptor on the outer nuclear membrane, and gray ones denote receptor in the endoplasmic reticulum.
(D) Comparison of nuclear Ca2+ signals to LTC4 in a control cell and in one in which the cytoplasm and nucleoplasm had been loaded with EGTA via the form EGTA-AM.
(E) Loading the cell with EGTA reduces NFAT4-cherry nuclear migration in response to LTC4. Migration can be rescued by stimulating cells with ionomycin (2 μM; 20 min).
(F) Aggregate data from several experiments as in (E) are summarized. Open bar above EGTA-AM denotes the extent of rescue by ionomycin. Data are represented as mean ± SEM.
(G) Graph plots NFAT4-cherry nuclear accumulation against the integrated nuclear Ca2+ signal in control cells and cells loaded with EGTA. Each point depicts NFAT4 movement and integrated nuclear Ca2+ from the same cell. The y axis represents peak NFAT nuclear/cytoplasmic ratio after stimulation with LTC4 minus the NFAT nuclear/cytoplasmic ratio before stimulation.
(H) Nuclear Ca2+ oscillations evoked by LTC4 run down quickly when Ca2+ entry through CRAC channels is blocked with 10 μM BTP2.
(I) Estimated nuclear Ca2+ concentration for an oscillatory response in a control cell is compared with a plateau-type response in an EGTA-loaded cell, following stimulation with 160 nM LTC4. To calibrate the nuclear Ca2+ signal in intact cells, following expression of NGCaMP3, Rmin and Rmax were obtained by stimulating cells with 5 μM ionomycin either in Ca2+-free external solution (with fluorescence measured ∼15 min later) or 10 mM Ca2+ external solution, respectively. The KD for nuclear GCaMP3 was taken as 660 nM from the literature. The estimated Ca2+ concentrations are, at best, only an approximation (see text). Arrow denotes LTC4 application.
(J) The graph plots the predicted build-up of nuclear Ca2+-calmodulin-calcineurin following stimulation with LTC4 in a control cell (red trace) and in one loaded with EGTA (blue trace).
(K) Contour plot depicts active nuclear calcineurin as a function of nuclear Ca2+ concentration and total nuclear calcineurin available.
See also Figure S4, Table S1, and description of the model in Supplemental information.