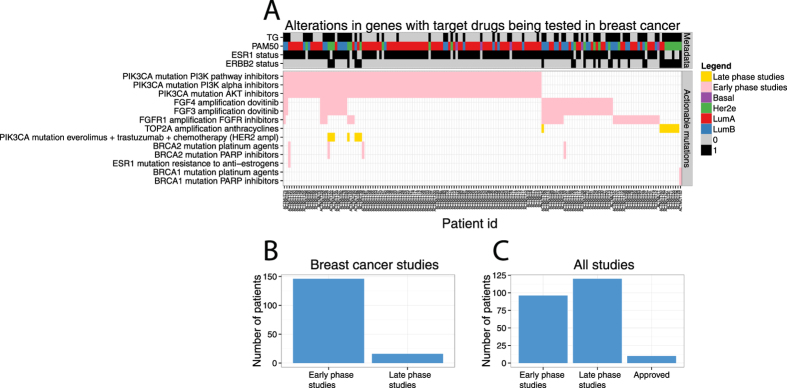
Figure 4. Overview of somatic alterations that are potentially actionable in the ClinSeq study.
(A) Each patient’s mutational profile was matched to the Dienstmann et al. knowledge base of actionable mutations including only breast cancer trials, excluding the ERBB2 amplification. Presence of a colored block in the intersection between an actionable mutation (rows in the heatmap) indicate a match between the somatic alterations in A patient (columns in the heatmap), Studies are classified as “Early” or “Late” from the Dienstmann et al. knowledge base31, also indicated by color. The top panel of display transcriptomic grade status (“TG”), molecular subtype (“PAM50”), ER status (“ESR1”) and HER2 status (“ERBB2”). (B) Summary of the number of patients in the present study that were potentially eligible for targeted drugs for indication breast cancer stratified by early and late phase studies. (C) Summary of the number of patients in the present study that were potentially eligible for targeted drugs for any indication, stratified by early and late phase studies as well as approved treatments.