Abstract
Study design:
A case report.
Objectives:
To report an unusual case of multiple spinal metastases from an undiagnosed well-differentiated liposarcoma (WDLPS) of the iliac wing and to stress the need of a meticulous clinical examination and further screening of patients with chronic and asymptomatic bony lesions.
Setting:
University of medicine of Monastir, Department of neurological surgery, Fattouma Bourguiba University Hospital, Monastir, Tunisia and University of Medicine of Tunis EL Manar, Department of neurological surgery, Tunisian National Institute of Neurology, Tunis, Tunisia.
Methods:
A 39-year-old man presented with signs of spinal cord compression for the past 2 weeks. His medical history was consistent for an asymptomatic right iliac wing mass that appeared 3 years ago and for which he has not consulted. Magnetic resonance imaging revealed multiple bony lesions of the thoraco-lumbar spine associated with a 6-cm right paravertebral mass at the T4 level extending posteriorly through the intervertebral foramina to the spinal canal causing major spinal cord compression. An emergent T2–T6 laminectomy allowed for a complete resection of the epidural mass. Pathological examination confirmed the diagnosis of well-differentiated liposarcoma. Adjunctive radiation therapy was administered.
Results:
The patient’s neurological status improved remarkably under an intensive care and rehabilitation program. He was ambulatory without assistance in the second postoperative week.
Conclusion:
The case reported in this paper represents a genuine example of the possible metastatic potential of WDLPSs of the bone and underscores the importance of examining patients thoroughly, especially when they have chronic and asymptomatic lesions.
Introduction
Liposarcoma (LPS) is an uncommon connective tissue tumor arising from lipoblast cells. Well-differentiated liposarcoma (WDLPS) represents the most frequent subtype. It mostly occurs in the limbs or retroperitoneum of elderly individuals and typically has no propensity to metastasize, unless it undergoes dedifferentiation.
To the best of our knowledge we present the first literature case of metastatic well-differentiated LPS of bone.
Case presentation
A previously healthy 39-year-old man presented to our emergency department with signs of spinal cord compression since the past 2 weeks that was complicated, 5 days prior to his admission, by incapacity in walking and sphincter disturbances. He also complained of severe back pain centered in his thoracic spine for the past 2 months.
His medical history was consistent for an asymptomatic right iliac wing mass discovered in March 2011 and for which he had not consulted. There was no history of trauma.
On admission, neurological examination revealed symmetrical spastic paraplegia with ankle clonus and extensor plantar responses in both sides, complete anesthesia with T4 sensory level, and urinary leakage. The physical examination, notably involving detailed clinical examination of the trunk and the extremities, showed a 6×8 cm mass depending on the right iliac wing.
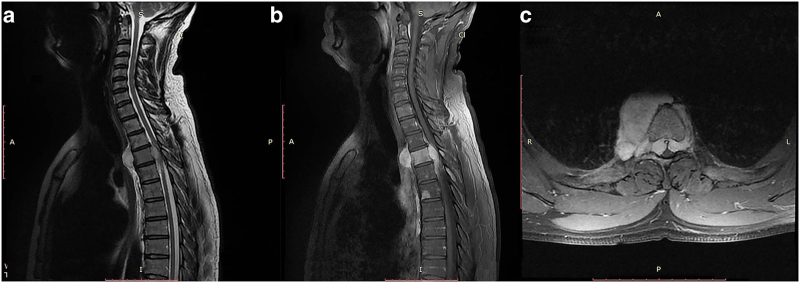
The chest X-ray, biological assay and electrocardiogram were normal. Magnetic resonance imaging showed multiple bony lesions of the thoraco-lumbar spine (Figure 1), associated with a 6-cm right paravertebral mass at the T4 level extending posteriorly through the intervertebral foramina to the spinal canal and causing major spinal cord compression (Figure 2).
Figure 1.
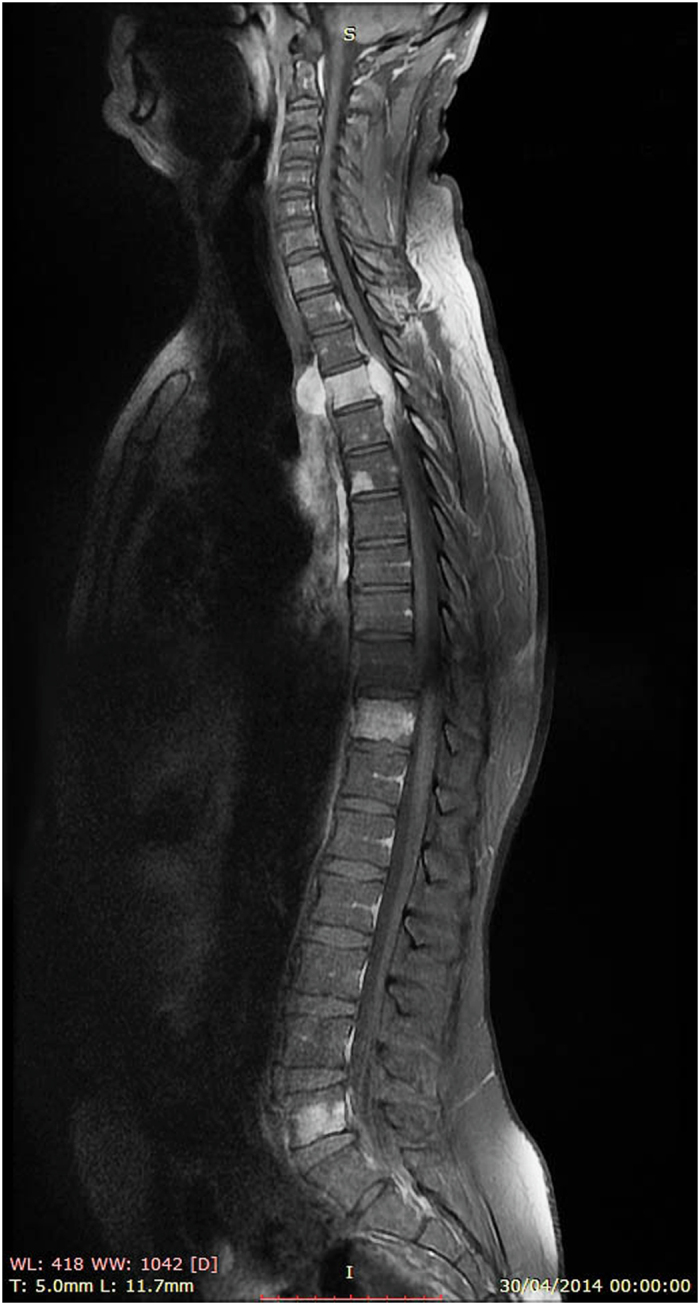
Sagittal T1-weigted post-gadolinium MR image showing multiple vertebral lesions of the thoraco-lumbar spine.
Figure 2.
T2-weighted (a) and T1-weighted post-gadolinium MR images (b, c) of the cervicothoracic spine showing the transforaminal extension of the pre-vertebral mass leading to a major spinal cord compression.
The patient was immediately transferred to the operative theater where a T2–T6 laminectomy aiming for spinal cord decompression and histological diagnosis was performed. The epidural portion of the tumor was prone to bleeding and, although hemostasis was difficult to achieve, progressive dissection allowed for its complete surgical removal. The T4 nerve root was entrapped by the tumor and was sacrificed.
Histological studies, performed at two different institutional centers, showed a densely cellular tumor containing several fields of adipocytic differentiation with clearly recognizable lipoblasts. These cells were well demarcated with abundant multi-vacuolated cytoplasm, and large irregular nuclei with dense chromatin and prominent nucleoli (Figure 3).
Figure 3.
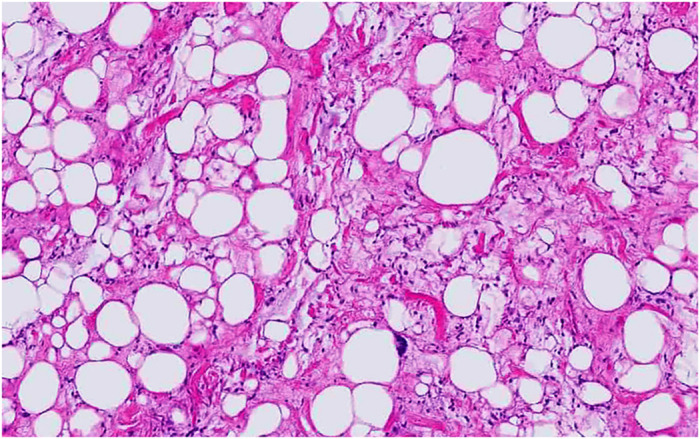
Photomicrograph of the tumor specimen showing typical features of well-differentiated liposarcoma with variably sized adipocytes and fibromyxoid stroma containing spindle cells with large, deep-staining nuclei.
Immunohistochemical staining was positive for vimentin, MDM2 and CDK4. It was negative for protein S100, Desmin, Myogenin, Smooth muscle actin, Caldesmon, CD68, HMB45, EMA and KL1 markers (Figure 3).
The final histological diagnosis was a WDLPS.
The patient was enrolled postoperatively under an intensive care and rehabilitation program. His neurological status remarkably improved and he was able to walk independently within 2 weeks postoperatively. The sphincter disturbances also disappeared.
Postoperative image-guided intensity modulated radiation therapy was administered at the tumor site and the patient was transferred to the orthopedic surgery department for removal of his iliac wing mass. His condition remained unchanged until the most recent follow-up examination 6 months later.
Discussion
LPS is an uncommon connective tissue tumor arising from lipoblast cells and mostly occurring in the thigh or retroperitoneum1–3 of elderly patients with a medium age at presentation of 49 years4 and a slight male predominance.3
It represents the most common soft-tissue sarcoma (20% of sarcomas in adults)5 and has an annual estimated incidence of 2.5 per one million inhabitants in population-based studies.6
Almost identical to the paradigm implemented by Enzinger and Winslow in 1962,7 the actual World Health Organization classification of tumors divides liposarcomas into five histological subtypes, among which the well-differentiated subtype (also described as atypical lipomatous tumor) is the most common, encompassing almost 50% of all cases.8
WDLPS is typically a locally aggressive, nonmetastasizing tumor that grows slowly and is rarely symptomatic.3 Men and women are equally affected and most patients are diagnosed in the sixth decade.9
Depending on the growth rate and the site of the tumor, WDLPS can attain a very large size at presentation, mainly in soft tissue and the retroperitoneal area. It can also localize in bone, but secondary involvement has never been previously reported unless the tumor undergoes dedifferentiation,10 a condition seen in only 6% of skeletal cases.10
In the present case, the patient’s medical history was significant for a 3-year progressively growing and indolent mass of his right iliac wing. The tumor reached a maximum size of 8 cm without pushing the patient to consult and in sharp contrast to that expected; our patient presented as an emergency case with signs of spinal cord compression, an uncommon pattern of presentation that outlines the unspecific shape of LPS and represents a genuine example of the possible metastatic potential of WD forms.
Radiologically, the sensitivity of bone scanning (16%) and positron emission tomography scanning (14%) is disappointing,11 and, although pathognomonic signs are still pending,12 magnetic resonance imaging appears to be the most reliable method for diagnosing spinal metastasis in patients with LPS.11 Spinal WDLPS may exhibit as a large encapsulated lipomatous mass with thick internal septations and may include focal nodules.13 A nodular focus of nonlipogenic tissue larger than 1 cm inside a WDLPS is suggestive of a DDLPS.13
Macroscopically, LPS tends to be well circumscribed or encapsulated, and usually shows a distinct lobulated pattern.1
Microscopically, the lipogenic origin of WDLPS can typically be seen with areas of lipoblastic differentiation paired with atypical stromal cells in the context of mature fat.3,10 It is characterized karyotypically by giant markers and ring chromosomes,14 which contain amplifications of oncogenes, such as MDM2 and CDK4 on chromosome 12q13–15.15
Nearly all cases of WDLPS overexpress MDM2 and CDK4,16 making them the most important markers for differentiating WDLPS from lipomas or other high-grade sarcomas and at the same time potential therapeutic targets in the upcoming era of personalized cancer therapy.10
As the best oncologic approach toward spinal metastases of LPS is not clear,17 patients should be managed by a multidisciplinary team with expertise in treating sarcomas. Like other well-differentiated sarcomas, WDLPS is usually treated by surgery alone when negative margins can be achieved and re-resection can be omitted in selected patients with positive margins when this procedure is associated with increased morbidity.18
In spinal metastasis, however, the feasibility and success of total resection is only seldom achieved and these kinds of tumors are usually chemoresistant;17 making radiation therapy of great adjunct in the multimodal management of such patients.17
In our case, surgery was advised in emergency for spinal cord relief and histological diagnosis; as our patient presented with multiple secondary lesions, only radiation therapy was considered postoperatively.
Prognostic data on LPS are mainly based on retrospective analyses from large sarcoma centers,10 and low-grade LPSs of the trunk are reported to have a 5-year disease-free survival of 95%.19
In the present report, we cannot postulate over the long-term prognosis of our patient, as metastatic forms of WDLPS have never been previously reported and the follow-up period is still too short. However, it seems clear that with such an aggressive behavior one cannot expect a good prognosis.
Recently, a multitude of compounds targeting MDM2 and CDK4 have entered early clinical development, which may soon change the landscape of systemic treatment of LPS and considerably improve the prognosis of patients harboring metastatic forms, like ours. However, before reaching that goal, it appears to be of paramount importance that the occurrence of spinal metastasis from a WDLPS of bone is recognized.
Conclusion
WDLPS of the bone can metastasize to the spine and present with signs of spinal cord compression.
Although its radiological appearance is somewhat unspecific, WDLPS should be considered in the differential diagnosis of secondary spinal tumors, especially if the patient already has an asymptomatic bony lesion.
As our understanding of the etiology and treatment of metastatic LPS continues to evolve, surgery, when feasible, plus radiation therapy seems to be actually a reasonable management policy for metastatic WDLPS of the bone.
The authors have no personal financial or institutional interest in any of the drugs, materials or devices described in this article. The authors declare no conflict of interest.
References
- Kirollos R, Koutsoubelis G, Ross S, Al Sarraj S. An unusual case of spinal metastasis from a liposarcoma. Eur J Surg Oncol 1996; 22: 303–305. [DOI] [PubMed] [Google Scholar]
- Tuoheti Y, Okada K, Miyakoshi N, Nishida J, Itoi E. Unusual variant of liposarcoma with multiple punctate calcifications. Skeletal Radiol 2002; 31: 666–670. [DOI] [PubMed] [Google Scholar]
- Mankin HJ, Mankin KP, Harmon DC. Liposarcoma: a soft tissue tumor with many presentations. Musculoskelet Surg 2014; 98: 171–177. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Enjoji M. Liposarcoma: a clinicopathologic subtyping of 52 cases. Acta Pathol Jpn 1982; 32: 933–948. [PubMed] [Google Scholar]
- Murphey MD. World Health Organization classification of bone and soft tissue tumors: modifications and implications for radiologists. Semin Musculoskelet Radiol 2007; 11: 201–214. [DOI] [PubMed] [Google Scholar]
- Kindblom LG, Angervall L, Svendsen P. Liposarcoma a clinicopathologic, radiographic and prognostic study. Acta Pathol Microbiol Scand Suppl 1975; 253: 1–71. [PubMed] [Google Scholar]
- Enzinger FM, Winslow DJ. Liposarcoma. A study of 103 cases. Virchows Arch Pathol Anat Physiol Klin Med 1962; 335: 367–388. [PubMed] [Google Scholar]
- Kim HS, Lee J, Yi SY, Jun HJ, Choi YL, Ahn GH et al. Liposarcoma: exploration of clinical prognostic factors for risk based stratification of therapy. BMC Cancer 2009; 9: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström K, Bergh P, Gustafson P, Hultborn R, Johansson H, Löfvenberg R et al. Liposarcoma: outcome based on the Scandinavian Sarcoma Group register. Cancer 2008; 113: 1649–1656. [DOI] [PubMed] [Google Scholar]
- Henze J, Bauer S. Liposarcomas. Hematol Oncol Clin North Am 2013; 27: 939–955. [DOI] [PubMed] [Google Scholar]
- Schwab JH, Boland PJ, Antonescu C, Bilsky MH, Healey JH. Spinal metastases from myxoid liposarcoma warrant screening with magnetic resonance imaging. Cancer 2007; 110: 1815–1822. [DOI] [PubMed] [Google Scholar]
- Hamlat A, Saikali S, Gueye EM, Le Strat A, Carsin-Nicol B, Brassier G. Primary liposarcoma of the thoracic spine: case report. Eur Spine J 2005; 14: 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005; 25: 1371–1395. [DOI] [PubMed] [Google Scholar]
- Fletcher CD, Akerman M, Dal Cin P, de Wever I, Mandahl N, Mertens F et al. Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol 1996; 148: 623–630. [PMC free article] [PubMed] [Google Scholar]
- Pedeutour F, Forus A, Coindre JM, Berner JM, Nicolo G, Michiels JF et al. Structure of the supernumerary ring and giant rod chromosomes in adipose tissue tumors. Genes Chromosomes Cancer 1999; 24: 30–41. [PubMed] [Google Scholar]
- Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol 2005; 29: 1340–1347. [DOI] [PubMed] [Google Scholar]
- Merimsky O, Kollender Y, Bokstein F, Issakov J, Flusser G, Inbar MJ et al. Radiotherapy for spinal cord compression in patients with soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 2004; 58: 1468–1473. [DOI] [PubMed] [Google Scholar]
- Canter RJ, Qin LX, Ferrone CR, Maki RG, Singer S, Brennan MF. Why do patients with low-grade soft tissue sarcoma die? Ann Surg Oncol 2008; 15: 3550–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan DC, Lewis JJ, Leung D, Brennan MF. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol 2000; 18: 1637–1643. [DOI] [PubMed] [Google Scholar]