Figure 8.
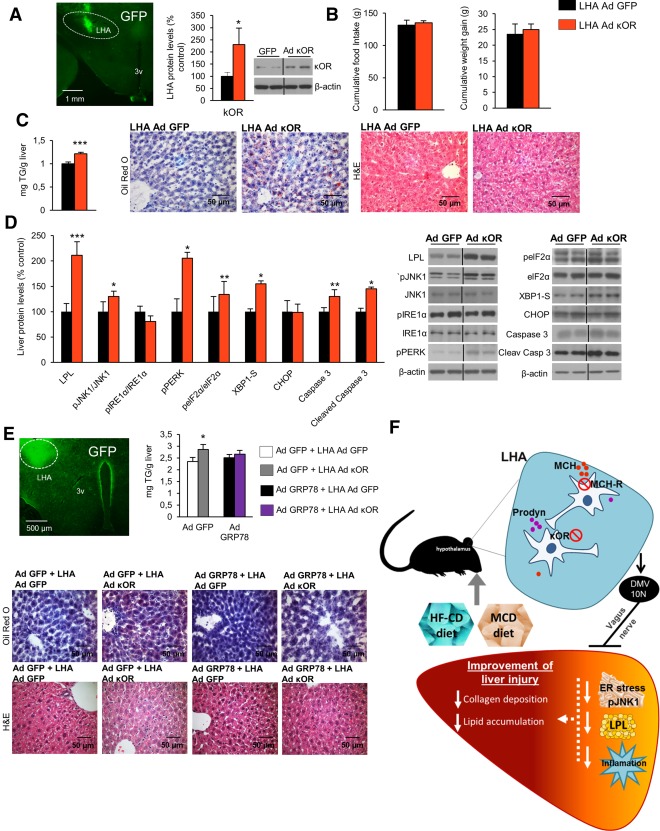
κOR overexpression in the LHA induces liver steatosis, and attenuation of hepatic ER stress blunts its effect. Representative photomicrograph of brain section showing the injection of adenoviral vector encoding GFP precisely placed in the LHA (×1.25 magnification) and κOR protein levels in the LHA of rats 1 week after injection of an adenoviral vector overexpressing κOR in the LHA of rats fed a chow diet (A). Effect of a 7‐day adenoviral overexpression of κOR in the LHA on food intake and body weight (B), liver TG content and oil red O and hematoxylin and eosin staining liver sections (C), and liver protein content of LPL, pJNK, JNK, pIRE1α, IRE1α, pPERK, peIF2α, eIF2α, XBP1S, CHOP, caspase 3, and cleaved caspase 3 (D). Representative photomicrograph of brain section showing the injection of adenoviral vector encoding GFP precisely placed in the LHA (×1.25 magnification), hepatic TG content, and representative photomicrographs of oil red O and hematoxylin and eosin staining of liver sections (×40 magnification) (E) in mice after tail vein administration of adenoviral vectors that overexpressed either GFP or GRP78 in the liver combined with overexpression of κOR in the LHA. Working model of the hypothalamic role of κOR in the control of liver metabolism (F). Protein β‐actin levels were used to normalize protein levels. Dividing lines indicate splicings within the same gel. Values are mean ± standard error of the mean of seven or eight animals per group. * P < 0.05, ** P < 0.01, *** P < 0.001 versus controls. Abbreviations: Ad, adenovirus; H&E, hematoxylin and eosin; 10N, vagus nerve; 3v, third ventricle.
