Figure 2.
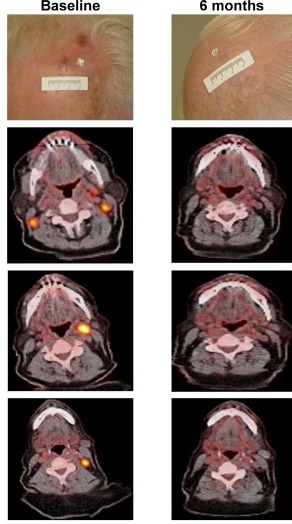
Representative images from a patient with stage IIIC disease randomized to talimogene laherparepvec who had a complete response. The patient was enrolled in the study with desmoplastic melanoma of the forehead with bilateral cervical fluorodeoxyglucose‐avid lymph nodes (left panel). Talimogene laherparepvec was injected only into the cutaneous lesion marked by the label (top row). At month 4, a partial response was reported and injection of talimogene laherparepvec was stopped. At cycle 6, a complete remission was reported that continued until the end of the study. Duration of response was 15.5 months. The patient was disease‐free at last follow‐up contact approximately 3 years after enrollment.
