Abstract
The aim of this meta‐analysis and systematic review of randomized controlled trials (RCTs) was to evaluate the efficacy and safety of adductor canal block (ACB) for early postoperative pain management in patients undergoing total knee arthroplasty (TKA). Relevant manuscripts comparing ACB with saline or femoral nerve block (FNB) in TKA patients were searched for in the databases of PubMed, EMBASE, and Cochrane library. The outcomes assessed included cumulative analgesic consumption, pain at rest or during movement, ability to ambulate, quadriceps strength, and complications (nausea, vomiting or sedation). For continuous outcomes, pooled effects were measured using weighted mean difference (WMD) or standard mean difference (SMD), together with 95% confidence intervals (CIs). For outcomes without sufficient data for synthesis, qualitative interpretation of individual studies was summarized. Finally, 11 RCTs involving 675 patients met the inclusion criteria. The pooled results showed that ACB resulted in less postoperative analgesic consumption than saline (WMD, −12.84 mg; 95% CI, −19.40 mg to −6.27 mg; P < 0.001) and less pain at rest or during activity. No conclusions could be drawn regarding ability to ambulate and quadriceps strength, because only one study reported these variables. Most studies comparing ACB and FNB reported similar effects on postoperative analgesic consumption (WMD, −0.56 mg; 95% CI, −8.05 mg to 6.93 mg; P = 0.884) and pain; however, ability to ambulate and quadriceps strength were significantly better with ACB (SMD, 0.99; 95% CI, 0.04–1.94; P = 0.041). Additionally, ACB did not increase the rate of complications. Our results suggest that, compared with saline, ACB decreases analgesic consumption and offers short‐term advantages in terms of pain relief. Compared with FNB, ACB was associated with better ability to ambulate and quadriceps strength.
Keywords: Adductor canal block, Analgesia, Meta analysis, Randomized controlled trials, Total knee arthroplasty
Introduction
Total knee arthroplasty (TKA) is a successful intervention for patients with painful degenerative diseases affecting the knee joint. The management of pain after TKA has always been a key focus in the clinical treatment of patients undergoing this procedure. Postoperative pain leads to decreased ability to mobilize the knee, prolonged hospitalization and increased complications. Despite comprehensive multimodal analgesic regimens, this problem has not been successfully addressed. Peripheral nerve blocks are increasingly preferred to relieve postoperative pain and to reduce opioid consumption and opioid‐related adverse effects in patients undergoing orthopedic procedures. Femoral nerve block (FNB) has been one of the most commonly used peripheral nerve blocks for managing post‐TKA pain. Compared with epidural or intravenous patient‐controlled analgesia alone, addition of FNB to an analgesic regimen provides superior pain control, reduces the incidence of postoperative complications and shortens the time to functional recovery1, 2. Nevertheless, since FNB targets the femoral nerve, which comprises both sensory and motor branches, it often weakens the quadriceps muscle, resulting in delayed mobilization and an increasing risk of falling3, 4.
Adductor canal block (ACB) is a relatively new type of peripheral nerve block technique introduced by Lund et al. 5. It offers better patient management after TKA than FNB5. ACB affects not only the two largest sensory contributors from the femoral nerve to the knee, namely, the saphenous nerve and the branch to the vastus medialis, but also the articular branches of the obturator nerve. However, the block is distal to most of the efferent branches to the quadriceps muscle and therefore largely preserves the strength of this muscle6, 7.
The efficacy of ACB compared with saline or FNB has been evaluated in several randomized controlled trials (RCTs), the results of which are controversial, being confounded by wide variations in study design. Dixit et al. examined the efficacy of ACB for all types of knee surgeries based on evidence obtained from 2000 to 2013 and obtained consistent results; the consistency of their results was attributable to the small sample size (only 289 patients) or the presence of bias8. Since their study, several new RCTs on this issue have been published, providing more evidence. We have therefore performed an updated systematic search and review of the available RCTs to assess the analgesic efficacy and safety of ACB for pain management after TKA.
Materials and Methods
Search Strategy
The databases of PubMed, Embase, and the Cochrane Library were searched using predefined search terms related to intervention (“adductor canal block” OR “adductor‐canal‐blockade” OR “ACB”) and operation type (“knee arthroplasty” OR “knee replacement” OR “Arthroplasty, Replacement, Knee”[Mesh]) for articles published from inception to February 2015, without any language restrictions. Additionally, the references of the included studies and pertinent systematic reviews were screened manually. Two authors (Jiang X and Wu CA) independently reviewed the titles and full texts of the relevant studies. Disagreements were resolved through discussion.
Inclusion and Exclusion Criteria
The inclusion criteria were (i) RCTs comparing ACB with FNB or a placebo; (ii) original studies conducted on TKA patients; and (iii) studies reporting at least one of the following outcomes: cumulative postoperative analgesic consumption, pain scores on a visual analog scale (VAS) or numeric rating scale (NRS) at rest or during 45° knee flexion, ability to mobilize, quadriceps muscle strength, opioid‐related adverse effects such as nausea, vomiting, and sedation and the success rate of the block. In the case of overlapping studies, the latest and/or most informative study was included. Exclusion criteria were defined as follows: (i) duplicate publications; (ii) studies with insufficiently reported data.
Data Extraction
The following items were extracted from each study: author details, publication year, study design, size of each group, characteristics of study subjects, type of intervention, type of systemic analgesia, pain at rest or during activity, opioid consumption in the early postoperative period and other outcomes. Data extraction was performed independently by two reviewers. Any discrepancies were resolved by discussion.
Quality Assessment
Risk of bias in individual studies was assessed based on the seven items in the Cochrane handbook; namely, random sequence generation, allocation concealment, blinding of participants or outcome assessors, incomplete outcome data, selective reporting and other bias9. Each item was recorded as “Yes,” “No,” or “Unclear.” “Yes” indicates a high risk of bias and “No” a low risk of bias. “Unclear” indicates a lack of information or an unknown risk of bias.
Statistical Analysis
In the case of outcomes for which the included studies provided sufficient quantitative data, weighted mean difference (WMD) or standard mean difference (SMD) and 95% confidence intervals (CIs) were used to estimate the overall effect. Evidence of statistically significant heterogeneity was assessed using the Q statistic, whereas the extent of observed heterogeneity was assessed using the I 2 statistic (ranging from 0% to 100%)10. In the absence of between‐study heterogeneity (I 2 ≤ 50%), the fixed‐effect model was used for data analyses. Otherwise, the random‐effect model was used. Publication bias was assessed with the Egger test11. Other outcomes such as pain at rest or during knee flexion and morphine‐related adverse effects were summarized using qualitative interpretations of the individual study methods and results because of variations in the time points and methods of measurements among the studies. All analyses were performed using Stata 9.0 (Stata Corp, College Station TX, USA). Statistical significance was assumed at a P value threshold of 0.05.
Results
The database searches identified 231 articles, of which 11 met the inclusion criteria and were used for qualitative and quantitative synthesis12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. Details of the process for screening published reports are shown in a flow chart (Fig. 1).
Figure 1.
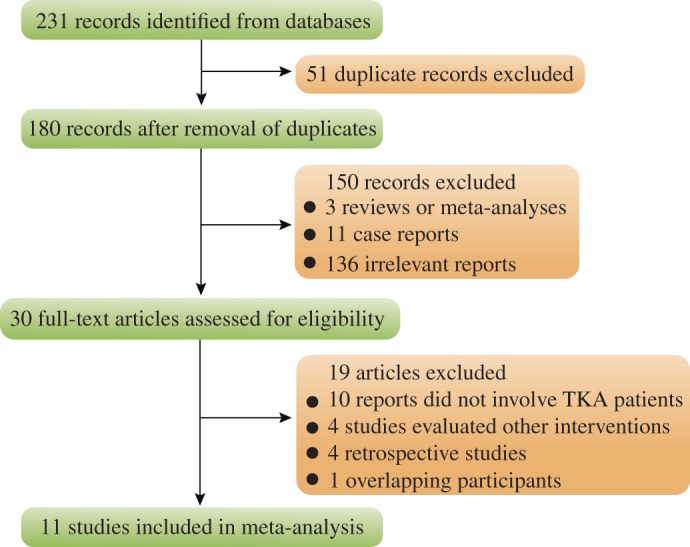
Flow chart showing selection of studies.
Study Characteristics
All 11 studies (involving 675 patients who underwent TKA) included for review were RCTs. The characteristics of these studies are summarized in Table 1. In brief, the control treatment was saline in four studies7, 12, 13, 15, FNB in six studies14, 18, 19, 20, 21, 22 and sham catheterization in one study16. For data analysis, we categorized the study with sham catheterization and the group of studies that used saline as including placebo treatment. Two studies examined the primary outcome soon after the operation (within 2 h of surgery)15, 22, whereas the other studies assessed it longer after TKA (6–48 h postoperatively)12, 13, 14, 16, 17, 18, 19, 20, 21. Ropivacaine was used as an anesthetic in the intervention group in nine studies12, 13, 14, 16, 17, 20, 21, 22 and bupivacaine in the other two studies18, 19. Nine studies used a VAS to evaluate pain12, 13, 14, 15, 17, 19, 20, 21, 22, whereas the other two studies used an 11‐point NRS16, 18. TKA was performed under spinal anesthesia in eight studies13, 14, 15, 18, 19, 20, 21, 22 and under general anesthesia in two12, 17. The type of anesthesia was not mentioned in the remaining study16. The studies' sample sizes ranged from 36 to 100 and the mean age of the study subjects from 34 to 71 years (Table 1).
Table 1.
Characteristics of the included studies
| Study | Time points | Country | Anesthesia | Interventions | Pain scale | Sample size (M/F, cases) | Mean age (ACB/control, years) | ||
|---|---|---|---|---|---|---|---|---|---|
| ACB | Control | ACB | Control | ||||||
| Jæger et al. (2012)12 | 0.5, 1, 2, 3, 4, 5, 6 h | Denmark | General anesthesia | 0.75% ropivacaine, 30 mL | Saline, 30 mL | VAS | 21 (12/9) | 20 (11/9) | 66/69 |
| Jenstrup et al. (2012)13 | 2, 4, 8, 24, 26 h | Denmark | Spinal anesthesia | 0.75% ropivacaine as a 30 mL bolus and additional 15 mL boluses at 6, 12, and 18 h postoperatively | Saline as a 30 mL bolus and additional 15 mL boluses at 6, 12 and 18 h postoperatively | VAS | 34 (18/16) | 37 (19/18) | 67/67 |
| Jæger et al. (2013)14 | 2, 4, 8, 24 h | Denmark | Spinal anesthesia | ACB with 30 mL bolus of 0.5% ropivacaine, followed by 0.2% ropivacaine infusion (8 mL/h) over the next 24 h | FNB with 30 mL bolus of 0.5% ropivacaine via a catheter, followed by 0.2% ropivacaine infusion (8 mL/h) over the next 24 h | VAS | 23 (5/18) | 27 (14/13) | 70/66 |
| Grevstad et al. (2014)15 | 15, 30, 45, 60, 75, 90 min | Denmark | Spinal anesthesia | ACB with 0.75% ropivacaine (30 mL) at 0 h and 30 mL isotonic saline at 45 min | Saline (30 mL) at 0 h, 0.75% ropivacaine (30 mL, ACB) at 45 min | VAS | 24 (5/19) | 25 (7/18) | 67/71 |
| Hanson et al. (2014)16 | 6, 12, 18, 24, 30, 36, 42, 48 h | USA | ‐ | Continuous ultrasound‐guided ACB with 0.2% ropivacaine at 8 mL/h with a 400‐mL reservoir | Continuous ultrasound‐guided ACB with sham catheter | NRS | 36 (11/25) | 40 (15/25) | 67/70 |
| Jenstrup et al. (2014)17 | 1, 2, 4, 6, 8, 24 h | Denmark | General anesthesia | ACB with ropivacaine for 24 h: 30 mL bolus of 0.75% ropivacaine, 15 mL bolus after 6 h, then 0.2% ropivacaine infusion (8 mL/h) | ACB with saline for 24 h: 30 mL saline bolus and 15 mL saline bolus after 6 h followed by saline infusion (8 mL/h) | VAS | 14 (8/6) | 16 (8/8) | 65/67 |
| Kim et al. (2014)18 | 6–8, 24, 48 h | USA | Spinal anesthesia | ACB with 0.5% bupivacaine (15 mL) and 5 μg/mL epinephrine | FNB with 0.25% bupivacaine (30 mL) and 5 μg/mL epinephrine | NRS | 46 (22/24) | 47 (18/29) | 68/67.6 |
| Memtsoudis et al. (2014)19 | 6–8, 24, 48 h | USA | Spinal anesthesia | ACB with 0.25% bupivacaine (15 mL) | FNB with 0.25% bupivacaine (30 mL) | VAS | 30 (26/33) | 29 | 64.41 |
| Shah et al. (2014)20 | 4, 8, 12, 24 h | India | Spinal anesthesia | ACB with 0.75% ropivacaine (30 mL) and 30 mL boluses of 0.25% ropivacaine at 4 h intervals till 8:00 am on the second day after surgery | FNB with 0.75% ropivacaine (30 mL) and 30 mL boluses of 0.25% ropivacaine at 4 h intervals till 8:00 am on the second day after surgery | VAS | 48 (13/35) | 50 (14/36) | 68.3/65.9 |
| Zhang et al. (2014)21 | 4, 24, 48 h | China | Spinal anesthesia | ACB with 0.33% ropivacaine (20 mL) | FNB with 0.33% ropivacaine (20 mL) | VAS | 30 (6/24) | 30 (8/22) | 63.7/61.9 |
| Grevstad et al. (2014)22 | 0, 30, 60, 90, 120 min | Denmark | Spinal anesthesia | “ACB” box contained 3 × 10 mL containers of 0.2% ropivacaine; “FNB” box contained 3 × 10 mL containers of isotonic saline | “ACB” box contained 3 × 10 mL containers of isotonic saline; “FNB” box contained 3 × 10 mL containers of 0.2% ropivacaine | VAS | 25 (7/18) | 25 (8/17) | 65/64 |
ACB, adductor canal block; FNB, femoral nerve block; M/F, number of males/number of females; MVIC, maximum voluntary isometric contraction; NRS, numeric rating scale; VAS, visual analogue scale.
The primary or secondary outcomes assessed in the studies included pain at rest and on mobilization, cumulative opioid consumption, quadriceps muscle strength and morphine‐related adverse effects and were measured at different time points after the operation. The risk of bias of the included studies is shown in Table 2. We considered that five trials had a low risk of bias12, 13, 14, 16, 17. In the remaining six studies, the risk of bias was unclear because of incomplete blinding or inadequate concealment of allocation (Table 2)15, 18, 19, 20, 21, 22.
Table 2.
Risk of bias in the included studies
| Study | Adequate sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias (similar systemic analgesia) |
|---|---|---|---|---|---|---|---|
| Jæger et al. (2012)12 | + | + | + | + | + | + | + |
| Jenstrup et al. (2012)13 | + | + | + | + | + | + | + |
| Jæger et al. (2013)14 | + | + | + | + | + | + | + |
| Grevstad et al. (2014)15 | ? | + | ? | ? | + | + | + |
| Hanson et al. (2014)16 | + | + | + | + | + | + | + |
| Jenstrup et al. (2014)17 | + | + | + | + | + | + | + |
| Kim et al. (2014)18 | + | + | + | ? | + | + | + |
| Memtsoudis et al. (2014)19 | ? | + | + | + | + | + | + |
| Shah et al. (2014)20 | + | ? | + | + | + | + | + |
| Zhang et al. (2014)21 | ? | ? | ? | ? | + | + | + |
| Grevstad et al. (2014)22 | + | + | ? | ? | + | + | + |
+, yes; ?, unclear.
Narcotic Consumption
Six RCTs, including 361 participants, reported the consumption of analgesics after surgery12, 13, 14, 16, 17, 18. An analysis of their results showed that ACB was associated with less analgesic consumption (WMD, −7.50 mg; 95% CI, −12.44 mg to 2.56 mg; P < 0.003). There was no significant heterogeneity among the studies. We performed subgroup analyses by different control groups. Pooled results from the ACB versus saline groups showed that ACB significantly decreased postoperative analgesic consumption (WMD, −12.84 mg; 95% CI, −19.40 mg to 6.27 mg; P < 0.001). However, the difference between ACB and FNB in postoperative analgesic consumption was not statistically significant (WMD, −0.56 mg; 95% CI, −8.05 mg to 6.93 mg; P = 0.884; Fig. 2).
Figure 2.
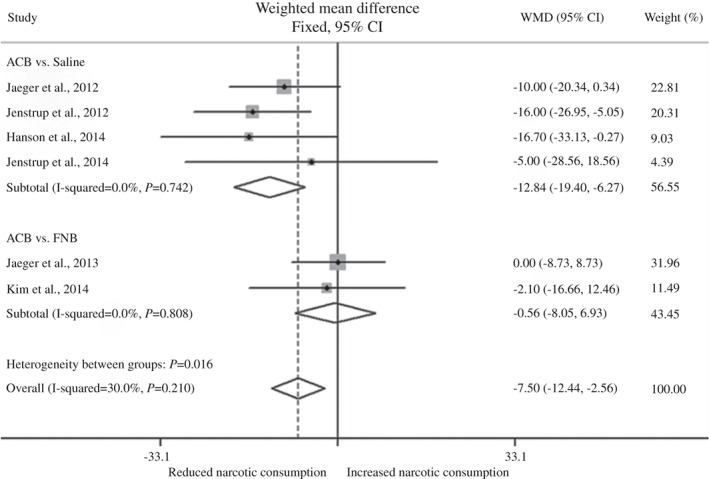
Forest plot showing relationship of different analgesic treatments with cumulative narcotic consumption after TKA. The diamonds indicate the overall effect as calculated using the weighted mean difference (WMD) in a fixed‐effect model.
For the sensitivity analysis, we evaluated the effect of excluding a study that included patients undergoing revision TKA; this did not materially change the results (data not shown)17. The Egger test for assessment of publication bias showed no statistically significant asymmetry (P = 0.636).
Pain at Rest and during Movement
Five randomized trials, including 283 subjects, compared the intensity of pain at rest between the ACB group and saline or no injection groups at different postoperative time points12, 13, 15, 16, 17. Compared with the control saline treatment or no intervention, ACB was associated with lower pain scores in the early postoperative period in all five studies; however, these differences were not statistically significant. The evidence from six RCTs indicated that ACB was not associated with more pain at rest than FNB14, 18, 19, 20, 21, 22 (Table 3).
Table 3.
Summary of the results of the included studies investigating the analgesic efficacy of ACB for TKA
| Study | Control treatment | Pain at rest | Pain during flexion | TUG test | Quadriceps muscle strength | Adverse effects | Success rate of ACB |
|---|---|---|---|---|---|---|---|
| Jæger et al. (2012)12 | Saline | −NS | − | − | NR | NS | 95% |
| Jenstrup et al. (2012)13 | Saline | −NS | − | −NS | NR | NS | 94% |
| Jæger et al. (2013)14 | FNB | −NS | −NS | NR | − | NS | NR |
| Grevstad et al. (2014)15 | Saline | − | − | NR | NR | NR | 98% |
| Hanson et al. (2014)16 | Sham catheter | −NS | − | NR | − | NS | NR |
| Jenstrup et al. (2014)17 | Saline | −NS | − | NR | NR | NS | NR |
| Kim et al. (2014)18 | FNB | +NS | NR | NR | − | NS | 98% |
| Memtsoudis et al. (2014)19 | FNB | +NS | +NS | NR | +NS | NR | NR |
| Shah et al. (2014)20 | FNB | −NS | −NS | − | NR | NS | 96% |
| Zhang et al. (2014)21 | FNB | NS | NS | NR | − | NS | NR |
| Grevstad et al. (2014)22 | FNB | +NS | −NS | − | − | NR | 100% |
−, results in favor of ACB and the difference is statistically significant; −NS, results in favor of ACB but the difference is not statistically significant; +NS, results in favor of the control group but the difference is not statistically significant; FNB, femoral nerve block; NS, the difference is not statistically significant; NR, not reported; TUG, timed‐up‐and‐go test.
Five randomized trials reported a difference in pain intensity during 45° flexion of the knee between the ACB and placebo groups12, 13, 15, 16, 17. ACB was associated with lower pain scores than placebo in the early postoperative period. However, the severity of pain during activity did not differ significantly between the ACB and FNB groups14, 18, 19, 20, 21, 22 (Table 3).
Other Outcomes
Ability to ambulate, as assessed by the timed up and go (TUG) test, was reported in four RCTs13, 14, 20, 22. The results from these studies showed that ability to ambulate was significantly better in the ACB than in the control group.
Quadriceps strength was assessed in six studies14, 16, 18, 19, 21, 22, five of which compared quadriceps strength in the ACB group with that in the FNB group14, 18, 19, 21, 22. Because these five studies used different measures and different units, we synthesized the data from them using the standard mean difference (SMD). The pooled results from the five studies suggested that ACB was associated with greater quadriceps strength than FNB (SMD, 0.99; 95% CI, 0.04–1.94, random‐effect model; P = 0.041; Fig. 3).
Figure 3.
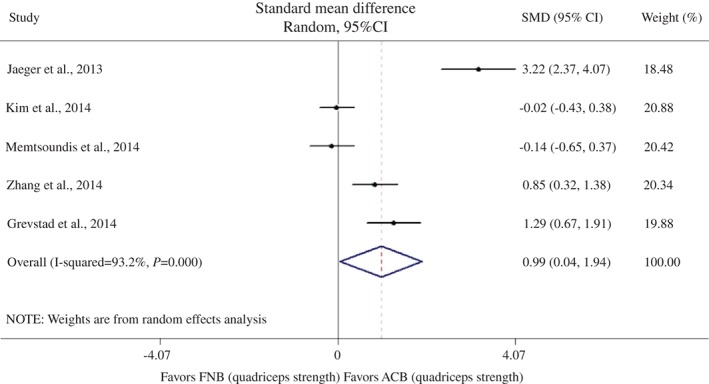
Forest plot of quadriceps strength and femoral nerve block (FNB) versus adductor canal block (ACB). The diamonds indicate the overall effect as calculated using the standard mean difference (SMD) in a random‐effect model.
No increase in the rate of nausea, vomiting or sedation was found in the ACB group in any of the included studies. Rather, Jaeger et al. reported that patients in the ACB group experienced significantly less nausea than those in the saline group12. Seven studies investigated the success rate of the block, which varied from 93.6% to 100% (Table 3).
Discussion
The present study summarizes the available evidence for the analgesic efficacy of ACB compared with saline administration, no injection or FNB. The pooled results show that ACB was associated with significantly less postoperative analgesic consumption than saline. In addition, ACB seemed to be associated with less pain at rest and during activity; however, these differences were not statistically significant. We were unable to reach a conclusion regarding the effects of ACB on ability to ambulate and quadriceps strength because only one study explored these issues. However, all the above findings indicate that provision of analgesia by ACB after TKA may offer some short‐term benefits over saline treatment or no intervention.
Most of the studies comparing ACB and FNB reported similar effects of the two blocks on postoperative analgesic consumption and pain scores. However, there was a statistically significant difference in favor of ACB for ability to ambulate and quadriceps strength. FNB is often used as a standard analgesic treatment after knee surgery23; however, it has been shown to reduce muscle strength, which may result in delayed mobilization and increase the risk of falling24. Unlike FNB, ACB is performed distal to the vastus medialis and is associated with less reduction in quadriceps muscle strength. Our pooled results confirmed this point. Furthermore, two studies reported that ACB was associated with greater ability to ambulate than FNB, as determined by the TUG test. Analgesic effects were similar in the ACB and FNB groups. Retrospective studies offer more evidence that the analgesic efficiency of ACB is comparable to that of FNB, and that ACB offers more benefits regarding postoperative ambulation25, 26, 27. Given all this, it seems that ACB is an attractive alternative to FNB in clinical practice.
The present study is an updated meta‐analysis to assess the efficacy and safety of ACB. One systematic review has previously been published on the benefits of ACB in knee surgery8. However, only three trials assessed in that review had investigated ACB for TKA; the primary outcome was opioid consumption in the first 24 h after surgery and no statistically significant differences were identified. The small sample size and potential bias prevented the authors from drawing any firm conclusions about the efficiency of ACB. In comparison, our review contains data from new, larger trials and was restricted to RCTs involving TKA patients. Despite these measures, the evidence we obtained was not strong enough to make definite clinical recommendations. Additionally, certain limitations should be considered. First, the outcomes were measured at different postoperative time points in different studies, and several of them could not be adequately synthesized by the meta‐analysis because there were insufficient data; this may have reduced the level of evidence. Therefore, in these measurements, large scale studies are necessary to draw firm conclusions about the effects of ACB. Secondly, although no statistically significant publication bias was found by the Egger test, we must emphasize that the exclusion of unpublished research may have affected the validity of our findings.
Conclusions
We found that ACB is associated with significantly less analgesic consumption after TKA than placebo. Nevertheless, the current evidence is not strong enough to conclude that ACB is superior to FNB; further well‐designed studies are required to address this issue.
Disclosure: The study was supported by the National Natural Science Foundation of China (No. 81330043; 81472139) and the Projects in the National Science & Technology Pillar Program during the Twelfth Five‐year Plan Period (No. 2012BAI10B02).
References
- 1. Hebl JR, Kopp SL, Ali MH, et al. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total hip arthroplasty. J Bone Joint Surg Am, 2005, 87 (Suppl. 2): S63–70. [DOI] [PubMed] [Google Scholar]
- 2. Allen HW, Liu SS, Ware PD, et al. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesth Analg, 1998, 87: 93–97. [DOI] [PubMed] [Google Scholar]
- 3. Charous MT, Madison SJ, Suresh PJ, et al. Continuous femoral nerve blocks: varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology, 2011, 115: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer M, Wang L, Onibonoje OK, et al. Continuous femoral nerve blocks: decreasing local anesthetic concentration to minimize quadriceps femoris weakness. Anesthesiology, 2012, 116: 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lund J, Jenstrup MT, Jaeger P, et al. Continuous adductor‐canal‐blockade for adjuvant post‐operative analgesia after major knee surgery: preliminary results. Acta Anaesthesiol Scand, 2011, 55: 14–19. [DOI] [PubMed] [Google Scholar]
- 6. Horn JL, Pitsch T, Salinas F, et al. Anatomic basis to the ultrasound‐guided approach for saphenous nerve blockade. Reg Anesth Pain Med, 2009, 34: 486–489. [DOI] [PubMed] [Google Scholar]
- 7. Kapoor R, Adhikary SD, Siefring C, et al. The saphenous nerve and its relationship to the nerve to the vastus medialis in and around the adductor canal: an anatomical study. Acta Anaesthesiol Scand, 2012, 56: 365–367. [DOI] [PubMed] [Google Scholar]
- 8. Dixit TA, Banerjee A, SK S. Efficacy of adductor canal block following knee surgery: a systematic review: 8AP5–4. Eur J Anaesthesiol, 2014, 31. [Google Scholar]
- 9. Higgins JP, Altman DG, Sterne JA. Assessing risk of bias in included studies In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester: John Wiley & Sons, Ltd, 2011. http://handbook.cochrane.org/ (accessed March 2011). [Google Scholar]
- 10. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med, 2002, 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 11. Egger M, Davey Smith G, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ, 1997, 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaeger P, Grevstad U, Henningsen MH, et al. Effect of adductor‐canal‐blockade on established, severe post‐operative pain after total knee arthroplasty: a randomised study. Acta Anaesthesiol Scand, 2012, 56: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 13. Jenstrup MT, Jaeger P, Lund J, et al. Effects of adductor‐canal‐blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand, 2012, 56: 357–364. [DOI] [PubMed] [Google Scholar]
- 14. Jaeger P, Zaric D, Fomsgaard JS, et al. Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: a randomized, double‐blind study. Reg Anesth Pain Med, 2013, 38: 526–532. [DOI] [PubMed] [Google Scholar]
- 15. Grevstad U, Mathiesen O, Lind T, et al. Effect of adductor canal block on pain in patients with severe pain after total knee arthroplasty: a randomized study with individual patient analysis. Br J Anaesth, 2014, 112: 912–919. [DOI] [PubMed] [Google Scholar]
- 16. Hanson NA, Cindy Jo A, Hostetter LS, et al. Continuous ultrasound‐guided adductor canal block for total knee arthroplasty: a randomized, double‐blind trial. Anesth Analg, 2014, 118: 1370–1377. [DOI] [PubMed] [Google Scholar]
- 17. Jaeger P, Koscielniak‐Nielsen ZJ, Schroder HM, et al. Adductor canal block for postoperative pain treatment after revision knee arthroplasty: a blinded, randomized, placebo‐controlled study. PLoS One, 2014, 9: e111951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology, 2014, 120: 540–550. [DOI] [PubMed] [Google Scholar]
- 19. Memtsoudis SG, Yoo D, Stundner O, et al. Subsartorial adductor canal vs femoral nerve block for analgesia after total knee replacement. Int Orthop, 2015, 39: 673–680. [DOI] [PubMed] [Google Scholar]
- 20. Shah NA, Jain NP. Is continuous adductor canal block better than continuous femoral nerve block after total knee arthroplasty? Effect on ambulation ability, early functional recovery and pain control: a randomized controlled trial. J Arthroplasty, 2014, 29: 2224–2229. [DOI] [PubMed] [Google Scholar]
- 21. Zhang W, Hu Y, Tao Y, et al. Ultrasound‐guided continuous adductor canal block for analgesia after total knee replacement. Chin Med J (Engl), 2014, 127: 4077–4081. [PubMed] [Google Scholar]
- 22. Grevstad U, Mathiesen O, Valentiner LS, et al. Effect of adductor canal block versus femoral nerve block on quadriceps strength, mobilization, and pain after total knee arthroplasty: a randomized, blinded study. Reg Anesth Pain Med, 2015, 40: 3–10. [DOI] [PubMed] [Google Scholar]
- 23. Paul JE, Arya A, Hurlburt L, et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta‐analysis of randomized controlled trials. Anesthesiology, 2010, 113: 1144–1162. [DOI] [PubMed] [Google Scholar]
- 24. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg, 2010, 111: 1552–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rasmussen M, Kim E, Kim TE, et al. A retrospective comparative provider workload analysis for femoral nerve and adductor canal catheters following knee arthroplasty. J Anesth, 2015, 29: 303–307. [DOI] [PubMed] [Google Scholar]
- 26. Mudumbai SC, Kim TE, Howard SK, et al. Continuous adductor canal blocks are superior to continuous femoral nerve blocks in promoting early ambulation after TKA. Clin Orthop Relat Res, 2014, 472: 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patterson ME, Bland KS, Thomas LC, et al. The adductor canal block provides effective analgesia similar to a femoral nerve block in patients undergoing total knee arthroplasty‐‐a retrospective study. J Clin Anesth, 2015, 27: 39–44. [DOI] [PubMed] [Google Scholar]


