Abstract
The ability to make artificial lipid bilayers compatible with a wide range of environments, and with sufficient structural rigidity for manual handling, would open up a wealth of opportunities for their more routine use in real‐world applications. Although droplet interface bilayers (DIBs) have been demonstrated in a host of laboratory applications, from chemical logic to biosynthesis reaction vessels, their wider use is hampered by a lack of mechanical stability and the largely manual methods employed in their production. Multiphase microfluidics has enabled us to construct hierarchical triple emulsions with a semipermeable shell, in order to form robust, bilayer‐bound, droplet networks capable of communication with their external surroundings. These constructs are stable in air, water, and oil environments and overcome a critical obstacle of achieving structural rigidity without compromising environmental interaction. This paves the way for practical application of artificial membranes or droplet networks in diverse areas such as medical applications, drug testing, biophysical studies and their use as synthetic cells.
Keywords: lipid bilayers, membranes, microfluidics, proteins, protocells
Droplet interface bilayers (DIBs)1, 2 represent a simple method for the production of artificial membranes where lipid bilayers are formed through the contact of aqueous droplets in an oil environment in the presence of lipid.1 The practical advantages of DIBs have widened the scope of membrane study and application3, 4 as well creating platforms for new single‐molecule studies in areas such as protein folding5 and DNA analysis.6 The droplet‐based membrane compartmentalization afforded by DIBs has made them attractive tools for the development of functional bio‐inspired devices such as power cells,7 synthetic tissue mimics,8 chemical logic platforms,9 and synthetic bioreactors.10 DIBs are increasingly representing a promising chassis for artificial cells,10, 11 affording lipid membrane barriers between spatially segregated compartments. However, the potential of such materials and their application to real‐world tasks is currently severely limited by an inherent fragility and challenges of interfacing to external environments. Villar and co‐workers12 demonstrated that a reduction of the continuous external oil volume to a discrete oil droplet [encapsulating the aqueous droplets] facilitated the formation of interface bilayers between the internal droplets and also between the internal and external environments12 (Figure 1 b). Such constructs, termed multisomes, have been recognized for their potential in medical, biotechnological, and industrial applications.13 However, such applications remain elusive as these constructs remain hampered by inherent fragility, with demonstration thus far limited to structurally supported droplets anchored on a wire loop or constrained within a microfluidic channel.14 As such, we propose a step‐change in the architecture of functional droplet networks, by embedding such constructs within a permeable, yet mechanically rigid shell (Figure 1 c–f).
Figure 1.
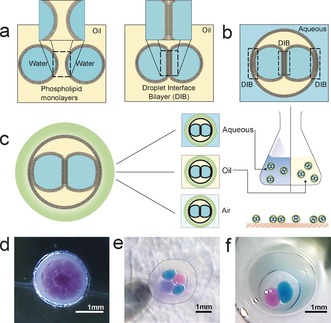
a) Schematic illustrating the formation of droplet interface bilayers (DIBs) from the contact of two droplets of water in oil in the presence of lipid. b) Formation of DIBs within a droplet of oil c) encapsulation of a droplet of oil containing DIBs in an alginate shell creates a robust freestanding structure compatible and communicable with a range of environments. d–f) Images of encapsulated DIBs (eDIBs) prepared in the laboratory with differing number and identity of internal aqueous cores.
Here we report the development of rugged, freestanding, mechanically stable, artificial bilayer systems comprised of networked droplets encapsulated in a hydrogel environment (Figure 1 c). We show that these encapsulated droplet interface bilayer (eDIB) constructs are stable in air, water, and oil environments, are long‐lasting and readily tolerate mechanical manipulation. We produce these novel constructs by microfluidic methods, with reliable control over the number of internal droplets and their composition. Importantly, these constructs possess the ability to communicate with the outside world through incorporation of membrane proteins. We anticipate that such developments will enhance the utility of DIBs by expanding their use beyond the laboratory into real‐world applications, as rugged, environmentally responsive smart materials.
The process for the creation of eDIBs is outlined in Figure 2. A hybrid 3D printed microfluidic device comprising sequential coaxial flow geometries15 enabled the double encapsulation of aqueous droplets. A T‐junction produced a regular stream of aqueous droplets in oil containing dissolved lipid (Figure 2 c). These droplets flowed within hydrophobic fluoropolymer (FEP) tubing which interfaced with the 3D printed microfluidic device. Another input channel in the device received a flow of an aqueous alginate solution with suspended CaCO3. This was delivered into a hydrophilic glass capillary embedded within the 3D printed device. The FEP tubing was mounted such that it terminated within this glass capillary, thereby creating a coaxial flow for droplet generation. This enabled the formation of individual droplets of oil within a continuous alginate flow, each containing a number of internal aqueous droplets (Figures 2 d, 3). The modulation of relative flow rates of water, oil, and alginate provided control over the number of internal aqueous cores and the frequency of production. A third fluid inlet in the 3D printed device received a continuous flow of mineral oil with dissolved acetic acid. This flowed into a second FEP tube within which the glass capillary carrying the double emulsion of water‐droplets‐in‐oil‐in‐alginate terminated. This second, hydrophobic, coaxial flow geometry, created the triple emulsion comprising a steady flow of alginate droplets, each containing an internal oil droplet further encapsulating aqueous droplets (Figures 2 e, 3).
Figure 2.
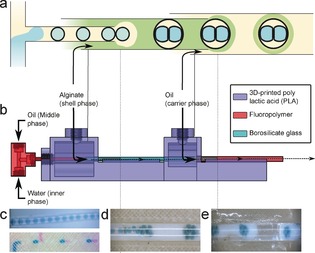
a) Illustration depicting the double coaxial microfluidic concept employed for the formation of eDIBs. b) Side‐view CAD schematic of the microfluidic device. Images of each successive droplet generation process where c) aqueous droplets in oil are created before d) segmentation of the oil phase within a continuous alginate flow creates discrete oil droplets containing aqueous droplet networks, e) subsequent segmentation of the alginate flow encapsulates these constructs in an alginate shell (eDIBs). Aqueous droplets can be formed by a single (c: upper panel) or double (c: lower panel) T‐junction geometry to define the internal contents of the aqueous droplets.
Figure 3.
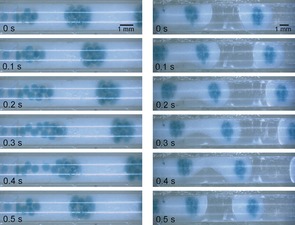
Time sequence images of the microfluidic manufacture of eDIBs. Double emulsions (left) are formed of aqueous droplets creating a DIB network within an oil droplet in a continuous alginate flow. At the second coaxial flow geometry, a triple emulsion is formed (right) creating an alginate shell around the bilayer construct (eDIB). Experimental flow rates: 0.196:0.196:2.5:6.67 mL min−1 (aqueous, oil, alginate, oil).
Lipid monolayers self‐assembled at the water–oil interfaces, gave rise to bilayers between contacting droplet interfaces. Alginate shell gelation and rigidification proceeded in‐flow, with the partitioning of acid from the mineral oil into the alginate solution, liberating calcium to gel the alginate. Constructs were collected on exit from the device. Unlike multisomes,12 the reported encapsulated structures were self‐supporting and resistant to rupture on contacting liquid air or container interfaces. Whilst providing structural rigidity, the permeable nature of the alginate shell also allows for diffusive access to the encased droplet bilayers.
Initially, the alginate shell appeared milky‐white, due to suspended CaCO3, with the capsule becoming transparent over a period of 15 minutes (see the Supporting Information). Excess CaCO3 in the alginate achieved rapid initial gelling, providing early structural rigidity whilst still in the microfluidic device. Off‐chip gelation and the formation of spherical constructs could also be achieved by delaying gelation (see the Supporting Information).
The use of a 3D printed double T‐junction to produce the internal aqueous droplets enabled the generation of alternating sequences of aqueous droplets of different composition (Figure 2 c). These droplets were then subsequently encapsulated to form eDIBs with control over the contents of individual internal compartments of the droplet network (Figure 1 f). In addition, modulation of the fluid flow rates provided a means to control the number of aqueous droplets encapsulated within each construct. Figure 4 e illustrates one to four internal droplets, with higher numbers illustrated in Figures 1 and 3. The microfluidic method developed here affords a continuous production method for eDIBs. Reproducibility was assessed at flow rates of 0.196:0.196:2.5:6.67 mL min−1 (aqueous, oil, alginate, oil). Here, eDIBs were produced at 2 Hz, with 96 % of constructs containing 10±1 cores (Figure 4 f) (n=100; see the Supporting Information).
Figure 4.
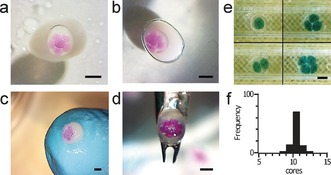
a–d) Encapsulated droplet interface bilayers (eDIBs) are compatible with a range of different physical environments and can withstand mechanical manipulation: a) eDIB in aqueous environment, b) eDIB in mineral oil, c) eDIB placed on a gloved finger and exposed to the air, d) eDIB manipulated with tweezers. e) Image demonstrating the formation of oil droplets with one, two, three and four internal aqueous cores. f) Histogram showing the reproducibility of production of constructs with higher numbers of internal cores (ca. 10; internal aqueous phase: 0.196 mL min−1; internal oil phase: 0.196 mL min−1; alginate phase: 2.5 mL min−1; carrier oil phase: 6.67 mL min−1, scale bars: 1 mm).
Alginate eDIBs were found to be stable in aqueous, oil, and air environments (Figure 4 a–d), as well as on solid surfaces including microscope slides, Petri dishes and sample tubes (see the Supporting Information). The constructs could be ejected directly from the microfluidic device into these environments, or easily manipulated between them by pipetting or tweezing, without damage to the internal droplet network (see the Supporting Information). We have found eDIBs are stable for weeks (see the Supporting Information). This ruggedness could enable long‐term membrane studies and facilitate otherwise inaccessible combinations of experiments and measurements on individual bilayer constructs. For example, co‐incubation of eDIBs with cell culture and extraction followed by microscopy should be possible. The ability to easily produce, store and handle constructs could enable off‐site production and application in a range of environments outside the laboratory. Alginate eDIBs provide an ideal platform for the application of droplet bilayer networks in a range of previously inaccessible environments owing to their structural rigidity, combined with the permeable properties of the hydrogel shell,16 and their externally facing bilayers. This permeability of the protective shell enables chemical diffusion to the internal droplet network, facilitating the possibility of communication between the internal cores of the eDIB and the wider environment. This is in contrast to previous efforts to stabilize DIBs involving the polymerization of the bulk oil phase to encase droplet bilayer pairs.17 Whilst this affords a mechanical scaffold, subsequent access to the bilayer or droplets is not possible.
The presence of lipid bilayers segregating compartments was confirmed by electrophysiology (Figure 5 a,b). A characteristic square wave current was recorded in response to a triangular wave potential, giving a bilayer capacitance of 2826 pF (bilayer area ca. 0.42 mm2 using a specific bilayer capacitance of 0.652 μF cm−2 for DPhPC4, 18). Additionally, transient electroporation of the bilayer was observed under an applied potential (+50 mV), giving rise to characteristic transient increases in current corresponding to the formation of electropores. In subsequent measurements, a 0.2 nL aqueous droplet containing the transmembrane pore‐forming protein, alpha‐hemolysin (α‐HL), was contacted with the eDIB. Under an applied potential of +30 mV, successive step‐wise increases in current were observed, as the α‐HL diffused through the alginate shell and individual pores spontaneously inserted into the bilayer. This resulted in an ion flux across the membrane (Figure 5 c,d). Two types of insertion events are observed. First, characteristic step increases in current associated with protein insertion into the bilayer directly separating the two electrodes (Figure 5 c inset red pore). Secondly, capacitive transient increases in current were observed that subsequently decayed (Figure 5 c blue trace). Such behavior has previously been reported as a result of pore insertion into indirectly interrogated bilayers of a wider droplet bilayer network.8, 19 Consequently, we attribute this behavior to insertions into neighboring bilayers of the connected network (Figure 5 c inset blue pore). Since pore insertion is a stochastic process, we observed a combination of these events with α‐HL pores inserting into a number of accessible, externally facing, bilayers of the network. At higher protein concentration, successive step‐wise increases in current with a broadening of conductance levels is seen, attributable to successive individual conductive insertions into the interrogated bilayer together with concurrent capacitive insertions (Figure 5 d). These experiments demonstrate the formation of bilayers within the eDIB and the ability of the internal droplets to communicate with the external environment, by the diffusion of functional protein through the alginate shell to the membrane, and subsequent ionic exchange between the internal cores and external environment. Droplet networks with membrane spanning channels have been shown to gain collective properties20 which should allow eDIBs to function as self‐contained signal processing units.
Figure 5.
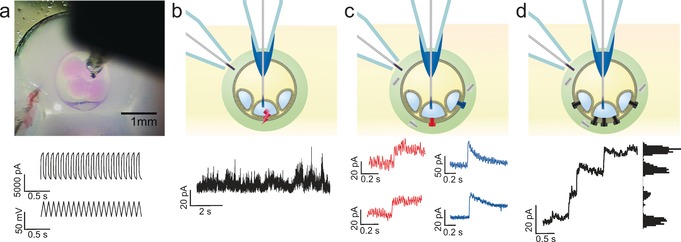
a) Image of eDIB with Ag/AgCl electrodes in the alginate shell and in an internal aqueous phase droplet (top). In response to a ±23 mV triangle wave, a capacitive current is measured, signifying the presence of a lipid bilayer (bottom). b) A short period of electroporation in an eDIB membrane is observed during a period under an applied potential of +50 mV. c) Following the contacting of the external shell of an eDIB with an aqueous droplet containing the protein pore, α‐hemolysin, protein insertion events are detected facilitating ion flux across the bilayer (red traces). Transient current spikes are also observed (blue traces) due to protein insertion into neighboring bilayers of the droplet bilayer network of the eDIB. d) At higher protein concentrations successive stepwise increases in current are measured as multiple protein pores insert (step size ca. 18 pA), with both direct (c: red traces) and indirect (c: blue traces) protein insertions measured.
Here, we have shown that encapsulated droplet networks, based on hierarchal triple emulsions, represent a highly robust artificial bilayer platform with the ability to interface with the external surroundings. eDIBs are able to withstand manual and mechanical handling and are stable for prolonged periods and in a range of environments. Microfluidic manufacture offers scalable production, and a reduction in channel dimensions should enable the production of smaller eDIBs. We have demonstrated that microfluidics provides a means to control both internal droplet number and contents. This control enables eDIBs to retain the favorable properties of DIBs, such as the asymmetry of droplet contents or bilayer lipid composition, the insertion of functional membrane proteins and the chemical communication between droplets, but whilst affording greater mechanical stability and environmental compatibility. This development provides the opportunity to widen the use of artificial lipid bilayers for fundamental science and also to harness the enormous potential of DIBs and droplet networks for use outside of the laboratory, enabling their application as functional materials for interfacing with the external world. Alginate is non‐immunogenic and has been used for protecting surgically implanted stem‐cells in tissue repair.21 eDIBs therefore could fulfill a similar role as synthetic organoids or as diagnostic or therapeutic platforms capable of dynamic interaction with the surroundings. We propose that such constructs will have use beyond healthcare with the opportunity to create lab‐in‐a‐capsule technology in compartmentalized eDIBs. These eDIBs could represent self‐contained assay platforms for use in complex environments that are not readily reduced to the laboratory setting. Furthermore, we anticipate that eDIBs will be valuable as an artificial cell‐like chassis in synthetic biology applications. Their mass production by microfluidics could enable them to be used as units of complex composition in higher‐order structures, forming synthetic tissues that are readily compatible with a wide range of environments.
Experimental Section
Full experimental details are provided in the Supporting Information. Briefly, a hybrid microfluidic device was fabricated by 3D printing. Aqueous droplets were formed by a single or double T‐junction and delivered to the 3D printed device. Coaxial flows for droplet encapsulation were engineered by interfacing FEP tubing and glass capillaries with the 3D printed device. Glass capillaries were silanized to render them permanently hydrophilic. Aqueous inner cores contained 50 mm sodium dihydrogen phosphate with sulphorhodamine B or lissamine green for color (50 μm). The central oil phase comprised hexadecane:silicone oil AR20 (1:1) with dissolved 1,2‐diphytanoyl‐sn‐glycero‐3‐phosphocholine (DPhPC) (5 mg mL−1). Liquid alginate (2 % w/v) with suspended CaCO3 particles (75 mg mL−1) formed the outer shell phase. The carrier oil phase was composed of mineral oil with 0.5 % glacial acetic acid. Electrophysiology: Custom Ag/AgCl electrodes were inserted into the internal aqueous cores and the alginate shell to probe the bilayer formed between the two phases. Bilayer capacitance and ion flux measurements were made, respectively, under applied potentials of ±23 mV triangular wave (10 Hz) or at a fixed potential of between ±10 and 50 mV.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Supplementary
Supplementary
Supplementary
Acknowledgements
O.K.C. is a Cardiff University SBP Research Fellow. A.J.L.M. and W.D.J. are funded by Cardiff Synthetic Biology Initiative. We thank Dr. Jin Li for initial multiphase emulsion work and helpful discussions.
D. K. Baxani, A. J. L. Morgan, W. D. Jamieson, C. J. Allender, D. A. Barrow, O. K. Castell, Angew. Chem. Int. Ed. 2016, 55, 14240.
References
- 1. Bayley H., Cronin B., Heron A., Holden M. A., Hwang W., Syeda R., Thompson J., Wallace M., Mol. BioSyst. 2008, 4, 1191–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leptihn S., Castell O. K., Cronin B., Lee E.-H., Gross L. C. M., Marshall D. P., Thompson J. R., Holden M., Wallace M. I., Nat. Protoc. 2013, 8, 1048–1057. [DOI] [PubMed] [Google Scholar]
- 3. Leptihn S., Thompson J. R., Ellory J. C., Tucker S. J., Wallace M. I., J. Am. Chem. Soc. 2011, 133, 9370–9375; [DOI] [PubMed] [Google Scholar]; Funakoshi K., Suzuki H., Takeuchi S., Anal. Chem. 2006, 78, 8169–8174; [DOI] [PubMed] [Google Scholar]; de Wit G., Danial J. S. H., Kukura P., Wallace M. I., Proc. Natl. Acad. Sci. USA 2015, 112, 12299–12303; [DOI] [PMC free article] [PubMed] [Google Scholar]; Barriga H. M. G., Booth P., Haylock S., Bazin R., Templer R. H., Ces O., J. R. Soc. Interface 2014, 11, 20140404; [DOI] [PMC free article] [PubMed] [Google Scholar]; Castell O. K., Berridge J., Wallace M. I., Angew. Chem. Int. Ed. 2012, 51, 3134–3138; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 3188–3192; [Google Scholar]; Gross L. C. M., Castell O. K., Wallace M. I., Nano Lett. 2011, 11, 3324–3328; [DOI] [PubMed] [Google Scholar]; Boreyko J. B., Polizos G., Datskos P. G., Sarles S. A., Collier C. P., Proc. Natl. Acad. Sci. USA 2014, 111, 7588–7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gross L. C. M., Heron A. J., Baca S. C., Wallace M. I., Langmuir 2011, 27, 14335–14342. [DOI] [PubMed] [Google Scholar]
- 5. Rodriguez-Larrea D., Bayley H., Nat. Nanotechnol. 2013, 8, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang S., Romero-Ruiz M., Castell O. K., Bayley H., Wallace M. I., Nat. Nanotechnol. 2015, 10, 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holden M. A., Needham D., Bayley H., J. Am. Chem. Soc. 2007, 129, 8650–8655. [DOI] [PubMed] [Google Scholar]
- 8. Villar G., Graham A. D., Bayley H., Science 2013, 340, 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.H. Yasuga, R. Kawano, M. Takinoue, Y. Tsuji, T. Osaki, K. Kamiya, N. Miki, S. Takeuchi in 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2013, 2013.
- 10. Elani Y., Law R. V., Ces O., Nat. Commun. 2014, 5, 5305. [DOI] [PubMed] [Google Scholar]
- 11. Elani Y., Biochem. Soc. Trans. 2016, 44, 723–730; [DOI] [PMC free article] [PubMed] [Google Scholar]; Dzieciol A. J., Mann S., Chem. Soc. Rev. 2012, 41, 79–85. [DOI] [PubMed] [Google Scholar]
- 12. Villar G., Heron A. J., Bayley H., Nat. Nanotechnol. 2011, 6, 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenstein M., Nat. Methods 2012, 9, 13–13;22312628 [Google Scholar]; Needham D., Nat. Nanotechnol. 2011, 6, 761–762. [DOI] [PubMed] [Google Scholar]
- 14. Elani Y., Solvas X. C. I., Edel J. B., Law R. V., Ces O., Chem. Commun. 2016, 52, 5961–5964. [DOI] [PubMed] [Google Scholar]
- 15. Utada A. S., Lorenceau E., Link D. R., Kaplan P. D., Stone H. A., Weitz D. A., Science 2005, 308, 537–541. [DOI] [PubMed] [Google Scholar]
- 16. Chan L. W., Lee H. Y., Heng P. W. S., Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar]
- 17. Jeon T.-J., Poulos J. L., Schmidt J. J., Lab Chip 2008, 8, 1742–1744; [DOI] [PubMed] [Google Scholar]; Venkatesan G. A., Sarles S. A., Lab Chip 2016, 16, 2116–2125. [DOI] [PubMed] [Google Scholar]
- 18. Taylor G. J., Venkatesan G. A., Collier C. P., Sarles S. A., Soft Matter 2015, 11, 7592–7605. [DOI] [PubMed] [Google Scholar]
- 19. Hwang W. L., Holden M. A., White S., Bayley H., J. Am. Chem. Soc. 2007, 129, 11854–11864. [DOI] [PubMed] [Google Scholar]
- 20. Maglia G., Heron A. J., Hwang W. L., Holden M. A., Mikhailova E., Li Q., Cheley S., Bayley H., Nat. Nanotechnol. 2009, 4, 437–440. [DOI] [PubMed] [Google Scholar]
- 21. de Vos P., Faas M. M., Strand B., Calafiore R., Biomaterials 2006, 27, 5603–5617; [DOI] [PubMed] [Google Scholar]; Tuch B. E., Hughes T. C., Evans M. D. M., Diabetes Metab. Res. Rev. 2011, 27, 928–932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Supplementary
Supplementary
Supplementary


