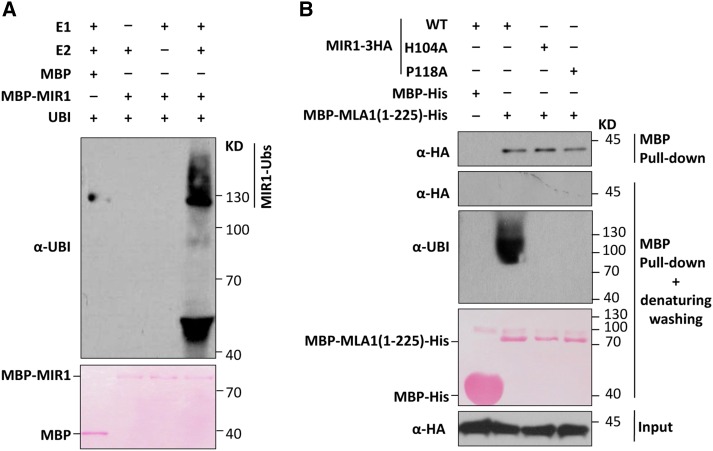
Figure 2.
MIR1 possesses E3 Ub ligase activity and can ubiquitinate the MLA1 N terminus. A, MIR1 displays E3 Ub ligase activity in the ubiquitination assay in vitro. Polyubiquitinated MBP-MIR1 was analyzed by immunoblotting. E1 (wheat GI:136632), E2 (AtUBC10), Ub (AtUBQ14), MBP, and the MBP-MIR1 (E3) fusion were all obtained from E. coli. B, MIR1 ubiquitinates the MLA1 N terminus in vitro. MIR1-3HA fusions were expressed in N. benthamiana by agroinfiltration, and MBP-MLA1(1-225)-His was obtained from E. coli. Crude extract of N. benthamiana was incubated with MBP-His or MBP-MLA1(1-225)-His protein before being mixed with Ni-NTA beads for His pull down. These mixtures were subjected to pull down and then immunoblot analysis of MIR1-3HA fusions (top) or further washing with denaturing washing buffer and immunoblot analysis of MIR1-3HA (middle) or ubiquitinated MBP-MLA1(1-225)-His (bottom). WT, Wild type.