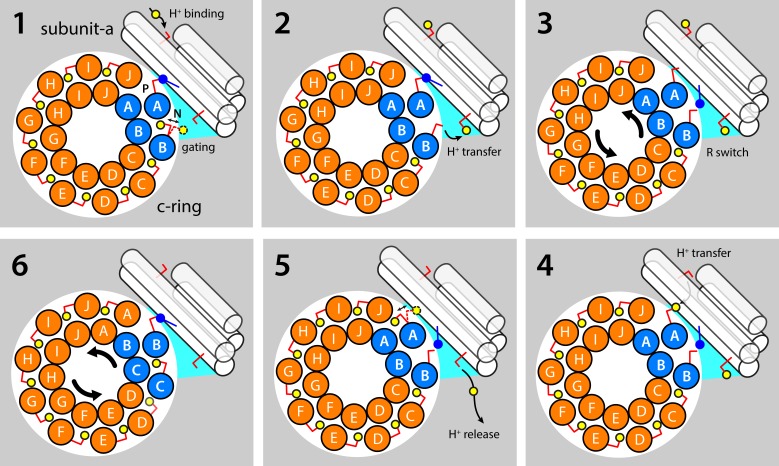
Figure 7.
Hypothetical mechanism coupling the translocation of protons through the a–c complex to the rotation of the c-ring. (1) Most of the c-ring binding sites are exposed to the membrane interior, and the conserved glutamate residue (Glu58 in Polytomella) is protonated and retracted in a closed state. However, one binding site is exposed to the aqueous crevice at the a–c interface that is open to the interior/negative side of the membrane; as a result, this N site fluctuates between a closed and open state. The adjacent c-ring binding site, counterclockwise, or P site, forms a salt bridge with a conserved arginine in TM4 of subunit a (Arg145). A protonatable side chain exposed to the exterior/positive side of the membrane serves as a transient buffering site for H+. (2) The aqueous nature of a crevice at the a–c interface (on the N side) fosters the deprotonation of the N site; the released H+ might be transiently captured by a buffering residue analogous to that on the opposite side of the membrane. (3) The N and P sites compete for the same interaction with Arg145, as the c-ring rotates back and forth, stochastically. (4) When the N site is engaged by Arg145, the proton buffered in the P channel can transfer to the deprotonated Glu58 in the P site, through an aqueous pathway. (5) The P site, H+ loaded, can now exchange between open and closed states. The H+ buffered in the N channel is released into the internal solution. (6) The protonated, closed P site enters the hydrophobic membrane interior, as the c-ring rotates counterclockwise; the N site becomes the new P site and continues to engage Arg145, and a new binding site enters the a–c interface, resetting the transport cycle. Note the proposed mechanism is entirely reversible, consistent with the fact that ATP synthases can function as ATP-driven ion pumps.