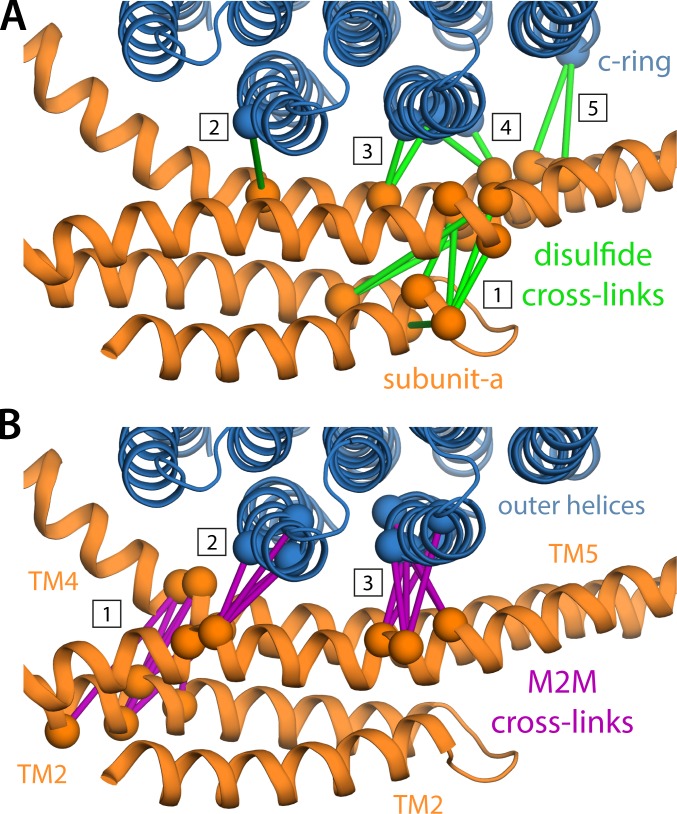
Figure 8.
Evaluation of the consistency of the proposed structure of the a–c complex with previous cross-linking studies of the E. coli ATP synthase. See Fig. S6 for a quantitative representation. (A) Green bars connect residues for which disulfide cross-links could be engineered, partially or fully, in the E. coli ATP synthase (see Table S2 and references therein). These cross-links cluster in five groups: cluster #1 pertains to the internal arrangement of the TM2-TM5 bundle, whereas clusters #2 to #5 reflect its orientation relative to the c-ring; note the latter are entirely consistent with the high tilt of TM5, relative to the outer helices in the c-ring. (B) Similarly to A, purple bars indicate cross-links between subunit a and the c-ring, mediated by an M2M spacer, which also cluster in three groups.