Figure 2.
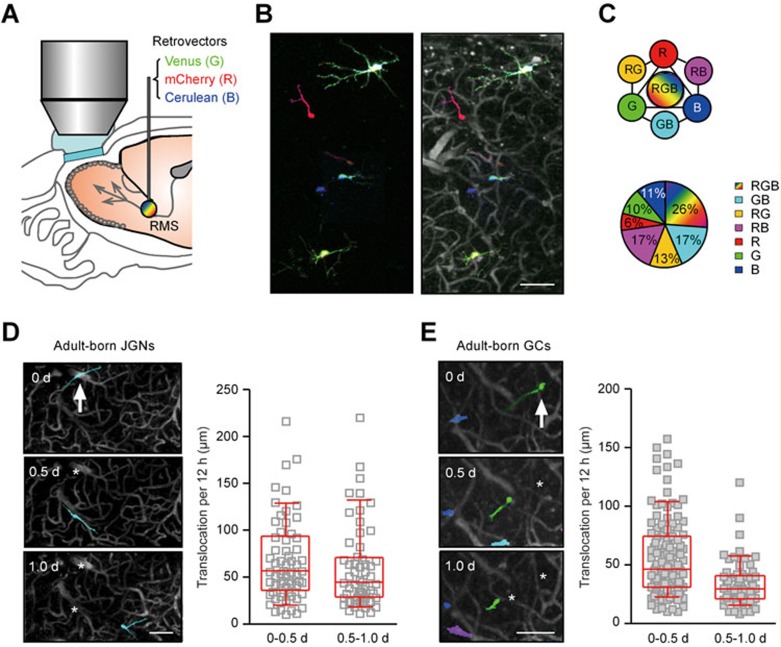
Visualization of the migratory behavior of adult-born neurons by oCPS. (A) Schematic drawing of the experimental setup. The mixture of concentrated retroviruses encoding the three fluorescent proteins (Venus as green, mCherry as red and Cerulean as blue) at the ratio of 1:1:1 in terms of infectious titer was injected into the mouse RMS for transduction of adult-born neurons in mice with chronic cranial windows. Transduced cells were imaged every 12 h starting from DPI 6 by means of two-photon microscopy. (B) Left: a MIP image (40-110 μm depth, step 2 μm) of RGB-labeled cells was generated by overlaying three images of basic colors red, green and blue acquired with the pre-determined settings (see Materials and Methods). Right: blood vessels were labeled with sulforhodamine B (i.p. injection) and the image of RGB-marked cells (left) was overlaid with blood vessel pattern to obtain a landmark reference for each cell. (C) Upper: schematic drawing illustrating the seven colors used for marking individual cells in this study. Lower: an example pie chart showing the fraction of adult-born neurons labeled by these colors (out of 111 cells from one of the 6 RGB-marked mice). (D) Left: a MIP image (70-120 μm depth, step 2 μm) of an adult-born JGN (arrow) migrating in the GL of the OB. Day 0 is the day of cell's arrival at the GL. Here and in E: asterisks mark previous locations of the cell. Right: plots showing the distance between the two consecutive positions of the cells during their first 12 h (0-0.5 d) or second 12 h (0.5-1.0 d) in the GL (translocation per 12 h; n = 65 cells, 5 mice). Here and in E: medians ± interquartile ranges are shown in red. (E) Left: a MIP image (216-336 μm depth, step 2 μm) of an adult-born GC (arrow) migrating in the GCL of the OB. Day 0 is the first day when adult-born GC was observed in the GCL. Right: the same graph as in D, but for data obtained from adult-born GCs (n = 141 cells, 5 mice). Scale bars, 100 μm.