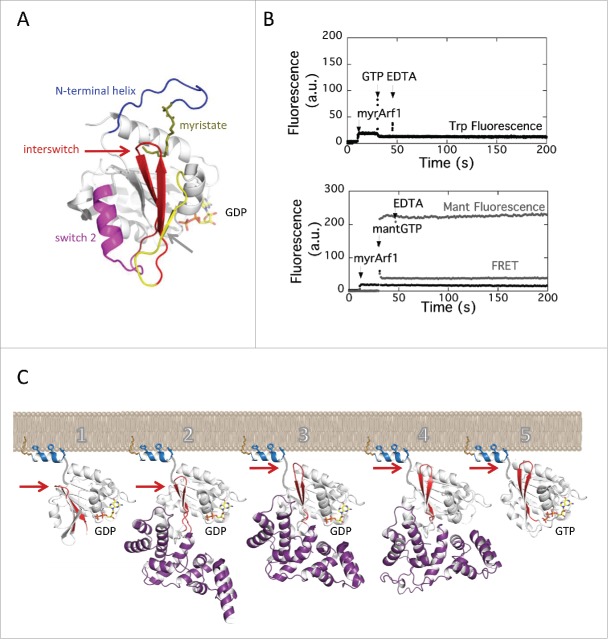
Figure 2.
Coupled activation by GTP and membrane recruitment of Arf GTPases. (A) The switch elements of Arf GTPases. The switch 1 and switch 2 are found in all small GTPases; the myristoylated N-terminal helix and the interswitch are switch elements unique to Arf and Arf-like small GTPases. The NMR structure of myristoylated yeast Arf1-GDP is from ref. 18; PDB entry 2K5U. (B) EDTA does not promote GTP binding on myrArf1 in solution. No nucleotide exchange was measured upon addition of EDTA, whether by tryptophan fluorescence (top) or by monitoring mant-GTP fluorescence (bottom) (see Methods for wavelengths). Analysis of mant-GTP fluorescence in the absence of protein showed that the large initial increase is due to its intrinsic fluorescence, and that the slow increase following EDTA addition is due to an effect of EDTA on the interactions between mant-GTP and Mg2+, as it was not observed in a Mg2+-free buffer (data not shown). (C) Schematic representation of the stimulation of GDP/GTP exchange by the Sec7 domain on membranes. One: the N-terminal helix, which locks Arf-GDP in an autoinhibited conformation in solution, is displaced by membranes; 2: membrane-attached Arf-GDP is recognized by the Sec7 domain (here, stabilized by the drug Brefeldin A); 3: the Sec7 domain promotes the toggle of the interswitch, which releases autoinhibition of the GTP-binding site and secures Arf to the membranes (here, stabilized by mutation of the catalytic glutamate to lysine); 4: the Sec7 domain dissociates GTP from Arf to form a nucleotide-free complex; 5: GTP binds to membrane-associated Arf. Arf-GDP is from ref. 11, PDB entry 1RRG; the Arf-GDP-Sec7 complexes trapped by Brefeldin A and by mutation of the catalytic glutamate are from ref. 26, PDB entries 1SD9 and 1R8S; nucleotide-free Arf-Sec7 is from ref. 14; Arf1-GTP is from ref. 86, PDB entry 1O3Y. The structure of the N-terminal helix and the position of the complexes with respect to membranes are dicative. The toggle of the interswitch is indicated by an arrow.