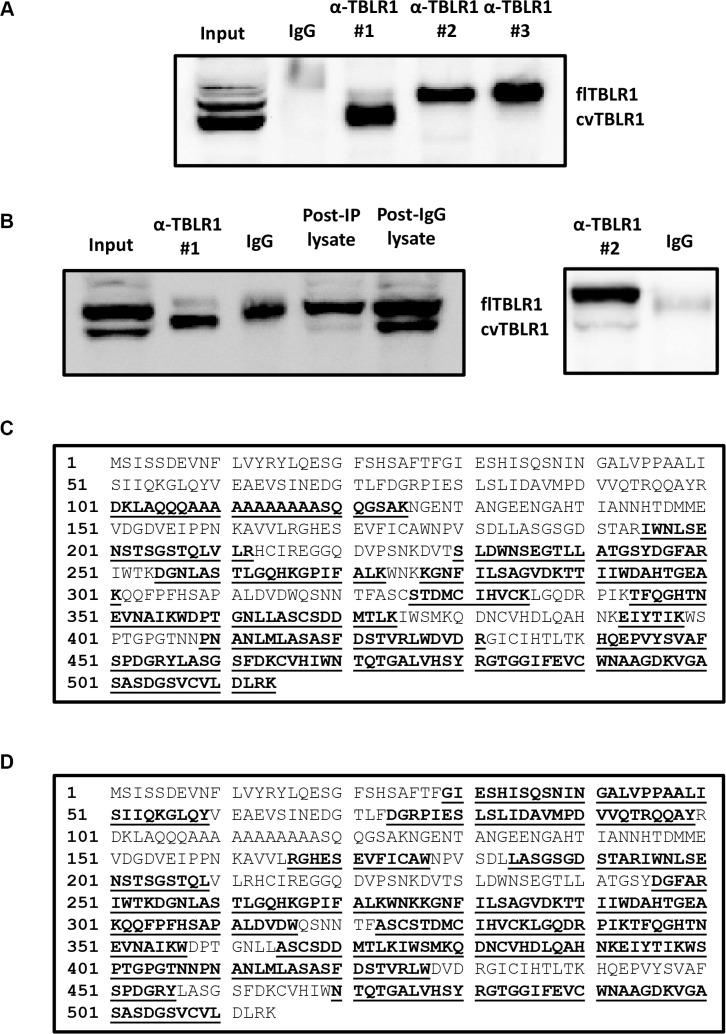
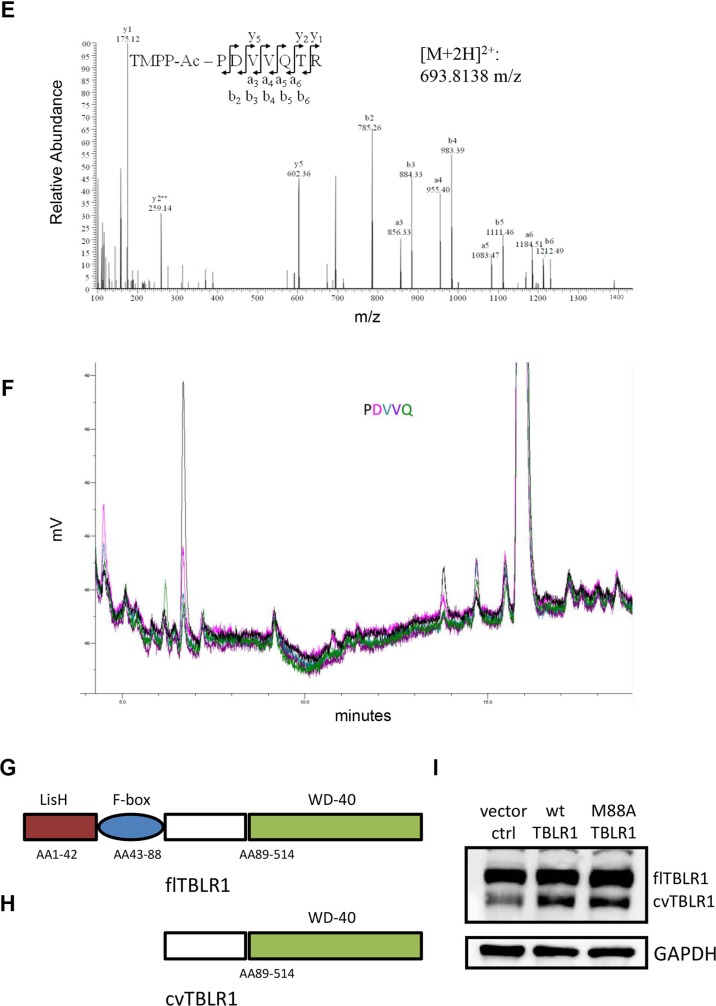
Figure 2. Sequence identification of cvTBLR1.
(A) Immunoprecipitation with three different TBLR1 specific antibodies to identify antibody to purify cvTBLR1. (B) Large scale purification of cvTBLR1 using antibody #1 and full length TBLR1 using antibody #2 for mass spectrometry (MS) sequencing. (C) TBLR1 sequence showing bold/highlighted sequences of peptides identified by MS from purified cvTBLR1. (D) TBLR1 sequence showing bold/highlighted sequences of peptides identified by MS from purified full length TBLR1. (E) Identification of N-terminal sequence of cvTBLR1 using TMPP labeled purified cvTBLR1 followed by MS sequence analysis. MS/MS spectrum of the doubly charged N-terminal peptide PDVVQTR of cvTBLR1 is shown, with the peptide sequence with annotation of the matched ions above the spectrum. (F) Confirmation of N-terminus of cvTBLR1 by Edman degradation analysis of the protein, overlay plot of the first five cycles. Y axis is millivolts. Absorbance is measured at 269 nm. Color code for the chromatogram matches the colors of the text reporting the identified amino acids. (G) Diagram of conserved domains in full length TBLR1. (H) Diagram of cvTBLR1 lacking the LisH and F-box domains of full length TBLR1 important for transcriptional regulation. (I) Overexpression of wildtype or mutated M88A full length TBLR1 shows increased cvTBLR1 in both conditions.