Figure 2. Everolimus inhibits phospho-Met phosphorylation via FKBP12.
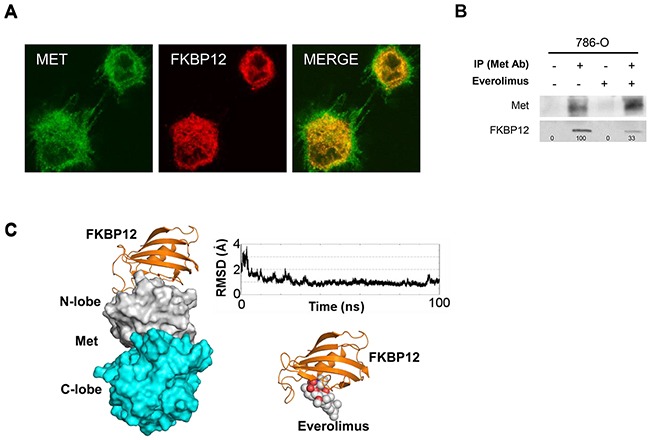
A. Colocalization analysis performed by immunofluorescence analysis: 786-O cells were grown on glass cover slips for 24 hours, then double-stained with anti-Met receptor and anti-FKBP12 primary antibodies and incubated with the appropriate rhodamine- or fluorescein-tagged goat anti-mouse or anti-rabbit antibody. B. Immunoprecipitation (IP) assay: 786-O cells, cultured in complete medium or treated for 24 hours with everolimus (0.5 μM), were immunoprecipitated using anti-Met antibody (Met Ab) and blotted with anti-Met and anti-FKBP12 antibodies. The same samples with normal IgG served as negative control. C. Computational analysis. Left: Calculated FKBP12/Met complex. FKBP12 is shown as orange ribbons while Met is shown as white and cyan surface for the N- and C-lobe, respectively. Top right: RMSD calculated for the FKBP12 backbone atoms along the 100-ns molecular dynamics simulation with respect to the FKBP12/Met average complex calculated over the 100 ns MD simulation. Bottom right: Everolimus/FKBP12 complex. The protein is shown as orange ribbons and the ligand as white and red spheres. The complex was calculated starting from the published X-ray rapamycin/FKBP12 complex.
