Figure 2. The procedure to measure the OXPHOS-linked oxygen consumption rate and glycolysis-linked proton production rate and the quality controls.
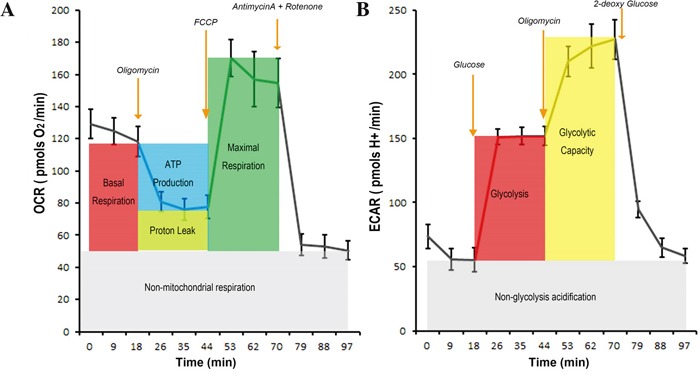
A. Measurement of OXHPHOS-linked oxygen consumption rate (OCR). H1299 Cancer cells were incubated in Seahorse-XF assay medium and the basal OCR value (summation of OXPHOS-linked OCR, the OCR associated with proton leak along respiratory chain, and the OCR irrelevant to mitochondial respiration) were obtained. When oligomycin A, an inhibitor of ATP synthase, was added, OXPHOS-associated OCR, but not the other two, was blocked. Addition of FCCP, a chemical uncoupler of electron transport and oxidative phosphorylation, maximized electron flow along the respiratory chain and the oxygen consumption. Finally, rotenone (an inhibitor of respiratory complex I) and antimycin (an inhibitor of respiratory complex III) were added to completely stop electron transfer along respiratory chain hence blocked OCR associated with ATP synthesis and proton leak. The remaining OCR was irrelevant to activity of mitochondrial respiration. B. Measurement of glycolysis-linked proton production rate (PPR). H1299 cancer cells were cultured in Seahorse-XF basal medium deprived of glucose and the basal proton production rate was obtained. Addition of glucose increased PPR and this increment represented glycolysis-associated PPR. Further addition of oligomycin maximized PPR because of Pasteur effect. Finally, 2-DG was added and the PPR was returned to the basal level hence glycolysis-associated PPR was confirmed. These procedures with proper quality controls were used to measure OXPHOS-associated OCR and glycolysis-associated PPR throughout this study.
