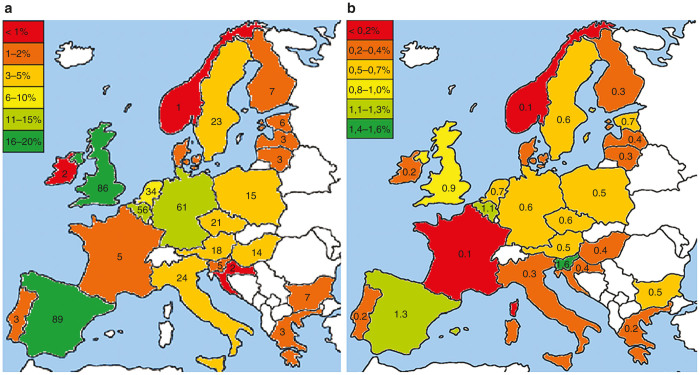
Figure 2.
Country-specific variations in gene- and cell-based therapy development. (a) Percentage of clinical trials with gene- and cell-based therapies per country derived from the public domain of the EudraCT database* depicted in color and absolute numbers of clinical trials performed per country (written in country). (b) Ratio of the number of clinical trials with gene- and cell-based therapy products performed in a country per the total amount of clinical trials in that country derived from the public domain of the EudraCT database. The white colored countries did not perform any studies with gene- and cell-based therapy products or do not belong to the European Union. (European map adjusted from http://www.youreuropemap.com/). * Since phase 1 clinical trials are not in the public domain of EudraCT and processing of trials in EudraCT can be delayed, clinical trials may be missing.