Abstract
Hirschsprung disease (HSCR) is characterized by absence of enteric neurons from the distal colon and severe intestinal dysmotility. To understand the pathophysiology and genetics of HSCR we developed a unique zebrafish model that allows combined genetic, developmental and in vivo physiological studies. We show that ret mutant zebrafish exhibit cellular, physiological and genetic features of HSCR, including absence of intestinal neurons, reduced peristalsis, and varying phenotype expressivity in the heterozygous state. We perform live imaging experiments using a UAS-GAL4 binary genetic system to drive fluorescent protein expression in ENS progenitors. We demonstrate that ENS progenitors migrate at reduced speed in ret heterozygous embryos, without changes in proliferation or survival, establishing this as a principal pathogenic mechanism for distal aganglionosis. We show, using live imaging of actual intestinal movements, that intestinal motility is severely compromised in ret mutants, and partially impaired in ret heterozygous larvae, and establish a clear correlation between neuron position and organised intestinal motility. We exploited the partially penetrant ret heterozygous phenotype as a sensitised background to test the influence of a candidate modifier gene. We generated mapk10 loss-of-function mutants, which show reduced numbers of enteric neurons. Significantly, we show that introduction of mapk10 mutations into ret heterozygotes enhanced the ENS deficit, supporting MAPK10 as a HSCR susceptibility locus. Our studies demonstrate that ret heterozygous zebrafish is a sensitized model, with many significant advantages over existing murine models, to explore the pathophysiology and complex genetics of HSCR.
Author Summary
Hirschsprung Disease (HSCR) is a common congenital intestinal motility disorder diagnosed at birth by absence of enteric neurons in the distal gut, leading to intestinal obstruction that requires life-saving surgery. HSCR exhibits complex inheritance patterns and its genetic basis is not fully understood. Although well studied by human geneticists, and modelled using mouse, significant questions remain about the cellular and genetic causes of the disease and the relationship between neuron loss and defective intestinal motility. Here we use accessible, transparent zebrafish to address these outstanding questions. We establish that ret mutant zebrafish display key features of HSCR, including absence of intestinal neurons, reduced gut motility and varying phenotype expressivity. Using live imaging, possible in zebrafish but not in mouse, we demonstrate that decreased migration speed of enteric neuron progenitors colonising the gut is the principal defect leading to neuron deficits. By direct examination of gut motility in zebrafish larvae, we establish a clear correlation between neurons and motility patterns. Finally, we show that mapk10 mutations worsen the enteric neuron deficit of ret mutants, indicating that mutations in MAPK10 may increase susceptibility to HSCR. We show many benefits of modelling human genetic diseases in zebrafish and advance our understanding of HSCR.
Introduction
The genetic basis of diseases exhibiting simple Mendelian inheritance can be readily uncovered, especially now using techniques available in the post-genomic era [1]. And, in the best examples, diseases such as cystic fibrosis and muscular dystrophy are well modeled using monogenic mouse mutants, enabling the diseases to be studied and new therapies to be tested [2, 3]. However, the genetic basis of human diseases exhibiting complex, multifactorial and/or polygenic inheritance is daunting to unravel. Additionally, conditions such as autism and schizophrenia, show spectra of phenotypes, making clear association between genotype and phenotype difficult [4]. Genetic studies of such diseases require advanced methodologies, including genome-wide association studies (GWAS), and this analysis throws up many possible candidate loci. And, perhaps expectedly, modeling these diseases has proven challenging (i.e. [5].
Hirschsprung Disease (HSCR), a common gut motility disorder (occurring in 1:5000 live births) characterized by defects in the enteric nervous system (ENS), is one such complex disease, being both multigenic and displaying varying expressivity [6, 7]. HSCR is diagnosed as an absence of enteric ganglia in varying extents of the distal colon, and affected newborns present with tonic contraction of aganglionic colonic segments, leading to accumulation of luminal contents and, if left untreated, toxic megacolon [8, 9]. Despite decades of genetic studies, twin challenges continue to drive research into studies of ENS development and the genetics of HSCR: firstly, understanding the developmental defects in the ENS that lead to intestinal aganglionosis that defines HSCR, and secondly, unraveling the complex genetics that control HSCR presentation.
The ENS is the part of the peripheral nervous system contained within the gut wall that controls intestinal motility, secretions and blood flow. Enteric neurons and glial cells are organized into interconnected ganglia situated between smooth muscle layers [10, 11]. The ENS derives mostly from vagal neural crest (NC) cells that invade the foregut during embryogenesis, hereafter called enteric NC-derived cells (ENCCs). Once within the gut wall, ENCCs proliferate extensively, migrate rostro-caudally and differentiate into vast numbers of neurons and glial cells organized into functional networks along the length of the gastrointestinal tract [12–14]. Overwhelming experimental evidence has demonstrated that the signaling pathway mediated by the RET receptor tyrosine kinase and the RET ligand GDNF, plays a critical role in ENS development, controlling survival, proliferation, migration and differentiation of ENCCs [12, 15–17]. Consistent with these findings, coding mutations of RET are detected in 50% of familial HSCR cases, while it has been suggested that non-coding regulatory mutations are likely present in most patients [8, 9]. Despite clear association between HSCR and RET, the cellular process(es) principally responsible for colonic aganglionosis is currently unknown. Furthermore, inheritance of HSCR is complex and not yet understood [8, 9]. For example, the non-coding mutations of RET associated with HSCR are common in the general population (~25%), indicating that they are not sufficient to lead to disease presentation [18]. Current models suggest that in most cases, individuals with non-coding RET mutations also carry modifier loci, many yet unknown, which contribute to HSCR presentation [18] and phenotype severity [6, 7]. Identification of such loci is a major challenge in HSCR research.
Transgenic mouse models have been critical in establishing key features of ENS development and HSCR disease [12, 13]. However several limitations are associated with these models, including a lack of simple heterozygous hypomorphic Ret mutations (as often observed in HSCR) phenocopying HSCR and it’s complex heritability, an inability to track ENS development in living embryos, an inability to examine in vivo intestinal motility in normal or disease states, and an absence of tractable models to screen for candidate HSCR susceptibility loci. To address these limitations, the genetically powerful and optically transparent free-swimming zebrafish larva has emerged as a viable alternative to mammalian models [19, 20]. Here we present an alternative model for HSCR disease in zebrafish and use it to address outstanding questions related to HSCR.
Results
Dose-dependent requirement for ret in ENS development
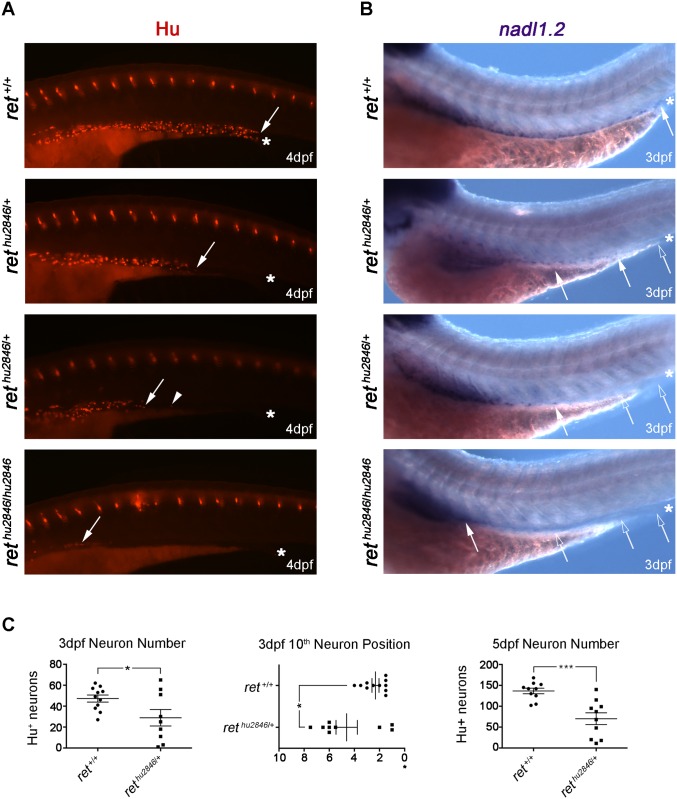
To establish a genetic model allowing us to explore Ret function throughout development, minimize experimental variation, and avoid potential off-target effects of Morpholino Oligos (MOs) [21], we used a TILLING strategy [22] to identify a zebrafish ret mutant allele. One allele, rethu2846, contains a point mutation in the 5’ ret coding sequences, leading to a truncated, non-functional protein [23]. We first assessed the effect of rethu2846 on gut colonization by enteric neurons. As expected, at 4dpf, HuC/D+ enteric neurons were detected in the intestinal bulb (a structure analogous to the stomach of terrestrial vertebrates) and along the full length of the intestine of WT (ret+/+) zebrafish larvae (Fig 1A) [19, 20]. In contrast, enteric neurons were almost absent from the gut of homozygous rethu2846/hu2846 larvae, which contained only a few HuC/D+ neurons in the intestinal bulb (Fig 1A). These genetic studies are consistent with findings of earlier MO experiments [24, 25], and confirm requirement of ret for ENS development in teleosts.
Fig 1. Loss of ret leads to dose-dependent reduction of ENS progenitors and neurons.
(A-B) WT (ret+/+), rethu2846/+, and rethu2846/2846 larvae immunostained at 4dpf with HuC/D antibody to visualize enteric neurons (A) and processed at 3dpf by RNA in situ hybridization to detect nadl1.2 expressing ENCCs (B). Asterisks indicate end of gut tube (anal pore), filled arrows and arrowheads denote position of last HuC/D+ neuron or nadl1.2+ ENCCs, and open arrows denote gut areas lacking nadl1.2+ ENCCs. (C) Number of HuC/D+ neurons in the distal gut was significantly reduced relative to WT counterparts, at 3dpf (WT: 47±3, rethu2846/+: 29±8, p = 0.0281) and 5dpf (WT: 137±7, rethu2846/+: 70±14, p = 0.0009). However, rethu2846/+ larvae had a phenotypic range: some rethu2846/+ larvae showed neuron numbers equivalent to WT, and other rethu2846/+ larvae showed comparably fewer neurons than WT. Position of the 10th most distal enteric neuron was also significantly altered in rethu2846/+ larvae. Y-axis indicates somite lengths from end of the gut (*) (in rethu2846/+ larvae 4.6±0.8 somite lengths from the end of the gut vs. 2.3±0.3 in WT, p = 0.012). Again, rethu2846/+ larvae display a phenotypic range.
Interestingly, in a cohort of rethu2846/+ larvae, enteric neurons were restricted to rostral mid-intestine regions (Fig 1A). By counting HuC/D+ enteric neurons in the distal gut of 3dpf larvae and 5dpf larvae, we found that on average the number of enteric neurons was significantly reduced relative to WT counterparts (Fig 1C). However, this phenotype was incompletely penetrant, and other individual rethu2846/+ larvae were less severely affected and, in some cases, were indistinguishable from WT (Fig 1C, S1 Fig). Indeed, several individual rethu2846/+ larvae had more neurons than the average observed in WT larvae (Fig 1C). To determine whether neurons of the distal gut were uniquely affected, or whether observed deficits represented a more general effect on the ENS cell population, we compared the position of the 10th most distal neuron between 3dpf rethu2846/+ larvae and WT larvae. In a percentage of rethu2846/+ larvae, the 10th neuron position was equivalent to WT, but in the remainder it was positioned more anteriorly (Fig 1C), indicating a generalized, incompletely penetrant ENS phenotype. The observed gut colonization defects of rethu2846/+ larvae contrast with the apparently normal colonization of the gut in heterozygous Ret mutant mice [26], and mirror colonic aganglionosis observed in patients with heterozygous RET mutations [27], and varying expressivity displayed by related individuals [6, 7]. This suggests that rethu2846/+ larvae could provide an animal model for studying the pathogenesis and complex genetics of HSCR.
ENCC migration deficits account for enteric neuron loss in rethu2846/+ larvae
To determine whether reduction or absence of enteric neurons in distal gut regions of rethu2846/+ and rethu2846/hu2846 larvae, respectively, is caused by abnormal gut colonization by progenitors or their defective neuronal differentiation, we analyzed gut colonization by migratory neural crest-derived cells using the independent ENCC marker nadl1.2 [24]. At 3dpf, nadl1.2-expressing cells were detected along the full length of the gut tube in all WT larvae analyzed (Fig 1B). In contrast, nadl1.2+ cells were found at varying positions in the gut of same stage rethu2846/+ larvae and were absent from the gut of rethu2846/hu2846 larvae (Fig 1B). Based on these experiments, we suggest that absence of differentiated neurons in the distal gut of ret mutant zebrafish reflects a failure of ENCCs to colonize the organ during development.
Defective gut colonization by ENCCs in rethu2846/+ larvae may be accounted for by reduced progenitor pool size, associated with decreased cell proliferation or increased cell death, or compromised migratory potential of ENCCs, or a combination of these factors. To distinguish between these mechanisms, we took advantage of the unique potential provided by zebrafish to directly visualize migration of ENS progenitors in vivo. First, we explored the possibility that a binary reporter system used to label other neuroectodermal lineages could be used to label ENCCs. By crossing SAGFF234A transgenic fish that express a Gal4 transcriptional activator gene trap construct [28, 29] with UAS:GFP transgenic animals [28], we were able to label several neural lineages within the central nervous system and cells within the gut. Closer examination revealed GFP expression in ENCCs and virtually all enteric neurons (S2 Fig). Using this tool, we then examined whether reduced proliferation and/or increased cell death may contribute to compromised ability of ENCCs to colonize the gut. BrdU incorporation (to label cells in S-phase of the cell cycle) demonstrated that at 48hpf proliferation of ENCCs was indistinguishable between rethu2846/+ and WT embryos (S3A–S3C Fig). Moreover no TUNEL+ cells were observed within the GFP expressing population of WT or rethu2846/+ embryos (S3F–S3I Fig). These observations suggest that the colonization defect observed in rethu2846/+ heterozygous larvae was unlikely to result from reduced size of the ENS progenitor pool.
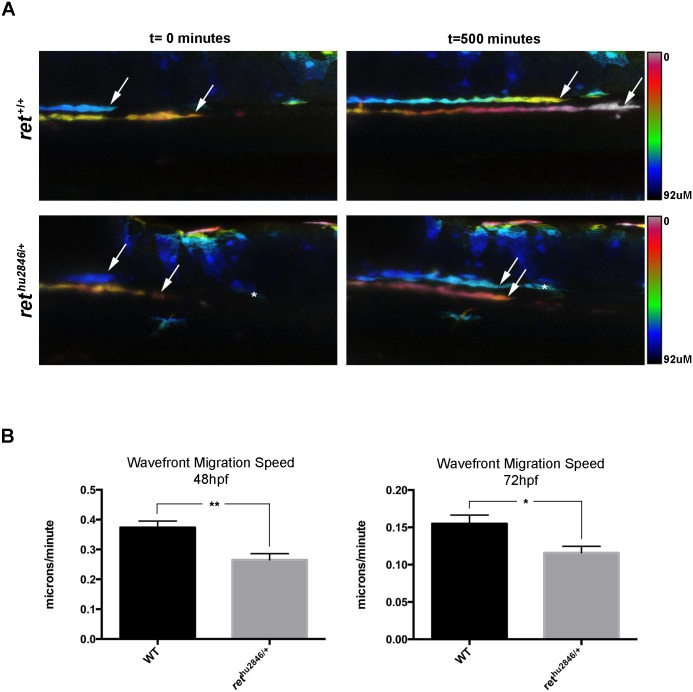
In the course of these studies, we noticed that the shape of the group of advancing ENCCs was more rounded and less elongated in rethu2846/+ embryos in comparison to WT embryos (S3D and S3E Fig), raising the possibility of a migratory deficit. We tested this idea directly by recording the migratory behavior of ENCCs using time-lapse confocal microscopy. We observed that at 48hfp, and 72hpf, the advancing speed of the front of the migratory population was reduced by 30% and 25%, respectively (Fig 2A and 2B, S1 and S2 Movies). We conclude that failure of enteric neurons to fully colonize rethu2846/+ zebrafish guts likely results from decreased migratory speed of the ENCC population during gut colonization.
Fig 2. rethu2846/+ larvae exhibit ENS progenitor migration deficits.
ENS progenitor migration at the front of migration (wavefront) visualized in real time by virtue of the SAGFF234A;UAS:GFP background labeling ENCCs. (A) Representative still images from confocal time-lapse recordings (depth-coded to resolve 2 migratory streams) of WT (ret+/+) and rethu2846/+ embryos at 0 minutes (48hpf) and 500 minutes (56hpf), show that distance travelled by the migration wavefront is decreased in rethu2846/+ larvae relative to WT larvae. Asterisk denotes a GFP+ cell from outside the ENS linage that remains in the same position. (B) The calculated wavefront migration speed (microns/minute) is significantly reduced in rethu2846/+ larvae relative to WT at 48–56hpf (p = 0.0022), and 72–80hpf (p = 0.0182).
Ret is required for normal intestinal motility
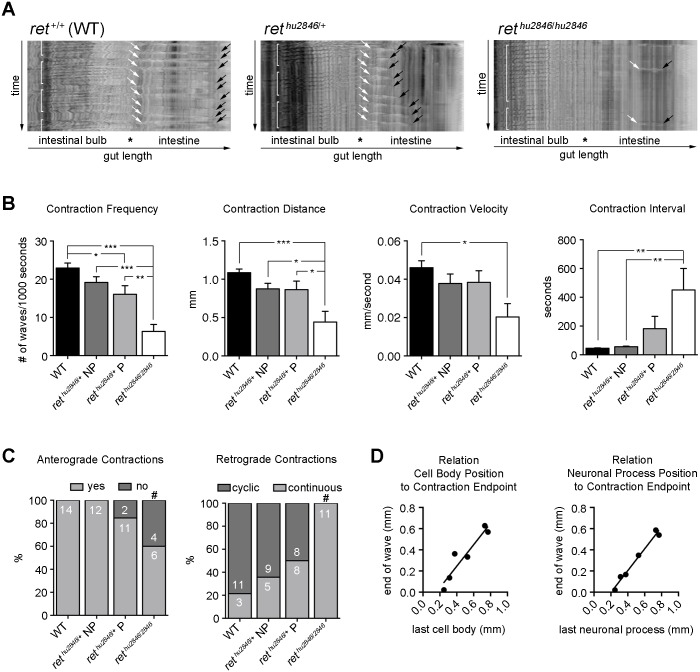
To examine the effect of ret mutations on the motor output of the ENS, gut motility was video-recorded in 7dpf larvae. In agreement with previous observations [30], in the intestinal bulb of WT larvae, we observed churning retrograde (anal to oral) waves, while in the intestine we observed anterograde (propulsive oral to anal) peristaltic waves (Fig 3A, S3 Movie). Importantly, the reproducible character of recorded intestinal motility patterns, displaying regular directionality and frequency, consistent travel distances and fixed speed (Fig 3A), allows their quantification and detailed assessment of the effects of ret mutation. rethu2846/hu2846 larvae showed severe defects in anterograde peristaltic activity in the intestine, including loss of contractions (Fig 3A and 3C, S5 Movie), reduction in frequency, distance, and velocity, and increase in interval (Fig 3B). Normal cyclical retrograde motility patterns were also lost in rethu2846/hu2846 larvae and motility in the intestinal bulb became nearly continuous (Fig 3C). These findings clearly indicate that presence of enteric neurons throughout the intestine is required for normal gut motility.
Fig 3. Intestinal motility is impaired in rethu2846/+ and rethu2846/2846 larvae.
(A) Spatiotemporal maps (STMs) generated to quantify 400 second recordings of gut motility in live 7dpf zebrafish larvae. WT (ret+/+) larvae exhibit cyclical retrograde (anal to oral) motility patterns in the intestinal bulb (brackets), and anterograde (oral to anal) peristaltic waves in the intestine starting at the intestinal bulb/intestine junction (white arrows); black arrows denote motility end points. STMs of rethu2846/+ larvae with ENS phenotypes and rethu2846/2846 larvae show alterations in cyclical retrograde motility patterns (brackets) and frequency and distance of anterograde motility waves (white to black arrows). (B) Anterograde contraction characterization. Phenotypic (rethu2846/+P) and non-phenotypic (rethu2846/+NP) larvae identified immunohistochemically. Reduced peristaltic frequency in rethu2846/2846 larvae and rethu2846/+ P relative to WT (one-way ANOVA, p = 0.0004; Bonferroni’s post-hoc, p = 0.0001 and p = 0.0367), and also reduced in rethu2846/2846 larvae as compared to rethu2846/+ NP (p = 0.0002) and rethu2846/+ P (p = 0.0035). Reduced contraction travel distance in rethu2846/2846 larvae relative to WT (one-way ANOVA, p = 0.0005; Bonferroni’s post-hoc, p = 0.0002), and relative to both rethu2846/+ P (p = 0.0232) and rethu2846/+ NP (p = 0.0237). Reduced contraction velocity in rethu2846/2846 relative to WT (one-way ANOVA, p = 0.0212; Bonferroni’s post-hoc, p = 0.0138). Increased contractions interval in rethu2846/2846 larvae relative to WT and rethu2846/+ NP (one-way ANOVA, p = 0.0078; Bonferroni’s post-hoc, p = 0.0053 and p = 0.0097). (C) Loss of anterograde contractions in rethu2846/hu2846 larvae compared to WT and rethu2846/+ NP (#, Fisher’s exact, p = 0.0198 and p = 0.028). rethu2846/2846 larvae show retrograde motility changes relative to all other genotypes, with contractions losing cyclic patterns (Fisher’s exact, vs. WT: p = 0.0001, vs. rethu2846/+ NP: p = 0.001, and vs. rethu2846/+ P: p = 0.0082). (D) Equivalent analysis in the SAGFF234A;UAS:GFP background, expressing GFP in ENS cells and their processes, enables mapping of contraction endpoints relative to cell body position (GFP+HuC/D+ cells, rs = 0.8857, p = 0.0333) and cell process (GFP+HuC/D+ process, rs = 0.942, p = 0.0167). Black lines indicate linear fits.
Next we analysed effects of reduced ret expression on gut peristalsis in rethu2846/+ larvae. Since the ENS gut colonization phenotype is variable in this genotype (S1B–S1D Fig), motility recordings were followed by immunostaining for HuC/D+ enteric neuron position. Spatiotemporal maps (STMs) of rethu2846/+ larvae with full colonization (non-phenotypic, NP; S1B Fig) were largely indistinguishable from those of WT animals (Fig 3B and 3C). In contrast, STMs of rethu2846/+ larvae with a clear colonization phenotype (phenotypic, P; S1C and S1D Fig) showed significant alterations in frequency of anterograde contractions (Fig 3B) and premature terminations of motility waves (Fig 3A, black arrows, S4 Movie). To determine more precisely the impact of neuronal loss on motility, we analysed rethu2846/+ embryos in the SAGFF234A;UAS:GFP background, allowing us to map the endpoints of anterograde contractions relative to position of the last GFP+HuC/D+ cell body or neuronal process (see S2E Fig). We observed a tight correlation between peristaltic endpoints and position of HuC/D+ cells and their processes along the gut (Fig 3D), further indicating the requirement of enteric neurons for normal gut motility. These findings, together with our failure to observe deficits in neuronal differentiation or subtype composition in rethu2846/+ larvae (S3K and S3M Fig), suggests that the altered motility patterns in rethu2846/+ larvae result primarily from failure of colonization of the distal intestine by ENCCs and associated reduction of enteric neurons. Taken together these studies demonstrate that the rethu2846/+ line provides an excellent model for analysing gut dysmotility associated with intestinal aganglionosis and HSCR.
mapk10 as a modifier gene for ENS development
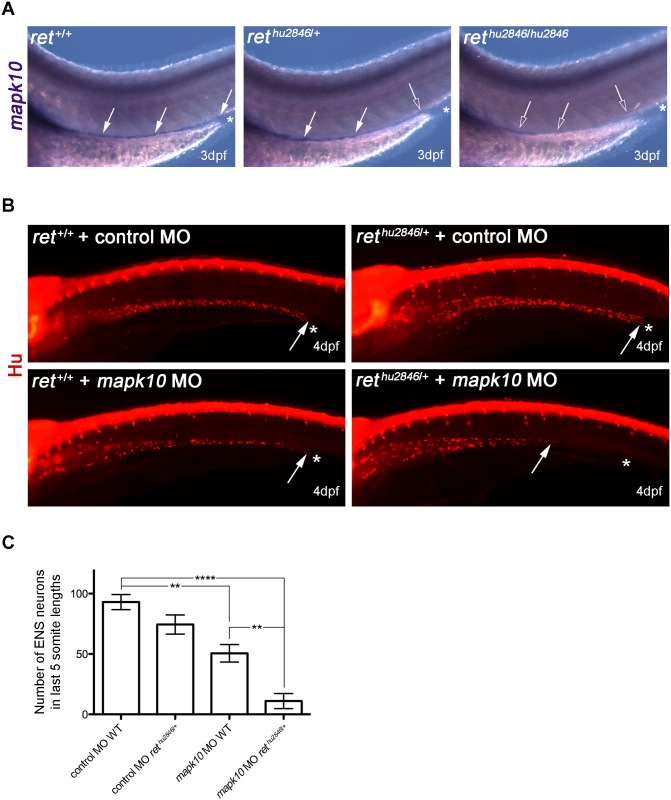
Although changes in RET signaling underlie almost all cases of HSCR, the vast majority of genetic changes associated with this locus represent non-coding, regulatory mutations [18]. Although such mutations are thought to increase susceptibility to HSCR, full expression of the disease phenotype requires interactions with mutations in unlinked modifier genes [18]. To test the idea that the rethu2846/+ genotype represents a sensitized genetic background suitable for phenotype-based screens for modifier loci affecting severity of ENS colonization phenotypes and manifestation of HSCR disease, we examined the effect of the mapk10 gene on the ENS phenotypes in rethu2846/+ larvae. Our focus on mapk10 was directed for several reasons. First, mapk10 was highlighted in a screen to identify novel vertebrate ENS markers and is highly expressed in the mammalian ENS [31]. Second, members of the Mapk family (also known as Jnks) act downstream of Ret in cancer cell signaling [32]. Third, Mapk signaling is impaired in RETS697A mutant mice and Mapk inhibitors block ENCC migration in cultured guts [33]. Finally, copy number variants in MAPK10 were identified in patients with HSCR [34]. By comparing the distribution of mapk10 transcripts in the gut of WT, rethu2846/+ and rethu2846/hu2846 larvae, we first established that similar to mammalian embryos [31], mapk10 is expressed in the zebrafish ENS (Fig 4A, see also S4 Fig). Next we performed gene knock-down experiments using a MO designed to block splicing of the mapk10 exon4-intron 4/5 boundary (mapk10MO) and generate a truncated protein lacking all known conserved functional domains (S5A Fig). mapk10MO injections were performed on embryos resulting from crosses between WT and rethu2846/+ animals, which was expected to generate WT and rethu2846/+ embryos in a 1:1 ratio. Relative to control MO, injection of mapk10MO into WT embryos led to a statistically significant reduction of enteric neurons in the gut of 4dpf larvae (Fig 4B and 4C), suggesting a role for mapk10 during ENS development. Interestingly, injection of mapk10MO into rethu2846/+ embryos resulted in dramatic reduction of enteric neurons relative to rethu2846/+ embryos injected with control MO or WT embryos injected with mapk10MO (Fig 4B and 4C). These results identify a role for mapk10 during ENS development and suggest a genetic interaction between mapk10 and ret, thus providing supporting for a model in which mutations in independently segregating loci modify RET activity and HSCR disease presentation.
Fig 4. Using rethu2846/+ as sensitized background to test role of mapk10 as ENS development modifier gene.
(A) RNA in situ hybridization shows mapk10 expression correlates with location of the ENS in WT (ret+/+), rethu2846/+, and rethu2846/hu2846 3dpf larvae (asterisks indicate end of gut, filled arrows indicate mapk10+ cells, and open arrows denote gut areas lacking mapk10+ cells). (B) MO gene knock-down of mapk10 (using a splice blocking MO, mapk10MO) performed on embryos from WT x rethu2846/+ cross to allow knock-down in both WT and rethu2846/+ embryos, and compared to a control MO. Immunostaining with HuC/D at 4dpf shows that most severe phenotypes are observed when mapk10MO is injected into rethu2846/+ embryos. Asterisks indicate end of gut tube (anal pore), and arrows denote position of last HuC/D+ neuron. (C) Quantification of neuron number in the last 5 somite lengths shows that injection of mapk10MO into WT embryos causes a modest, statistically significant reduction of enteric neurons in the gut relative to WT larvae injected with control MO (one-way ANOVA, p<0.001; Bonferroni’s post-hoc test, p = 0.0033), revealing a role for mapk10 in normal ENS development. Injection of mapk10MO into rethu2846/+ embryos causes severe reduction of enteric neurons, which is statistically different from either rethu2846/+ injected with control MO (one-way ANOVA, p<0.001, Bonferroni’s post-hoc test, P<0.0001) or mapk10MO injected into WT embryos (one-way ANOVA p<0.001, Bonferroni’s post-hoc test p = 0.006).
A novel mapk10 mutant zebrafish exhibits mild ENS colonization phenotypes
While MOs are invaluable screening tools, their use can lead to non-specific effects, confounding experimental interpretations [21]. For this reason, we employed genome-editing techniques [35] to generate a mapk10 mutant line and refine our analysis. Using TALEN [36], we generated, among other mutations, a 10 base pair deletion in exon 4 (mapk10Δ10) causing a frameshift in the open reading frame, leading to a premature stop codon and protein truncation, which lacks the entire protein kinase catalytic domain and all Mapk/Jnk conserved domains (S5B Fig). mapk10Δ10/+ and mapk10Δ10/Δ10 zebrafish were viable and fertile, without obvious morphological or behavioral phenotypes.
To further examine the role of mapk10 in ENS development, we compared enteric neuron number in the distal gut of 4dpf WT, mapk10Δ10/+ and mapk10Δ10/Δ10 larvae. Although no difference was detected between WT and heterozygous mapk10Δ10 larvae, mapk10Δ10/Δ10 larvae showed a relatively small, but statistically significant reduction in ENS cell number in the gut relative to WT (Fig 5A). In all genotypes, the percentage of animals containing a given range of enteric neurons in the distal gut follows a normal distribution (Fig 5B). Together, these observations are consistent with observed effects of mapk10MO injections and confirm a role for mapk10 in normal ENS development. While both mapk10 loss of function experiments show the same effect to reduce ENS neuron number, the mapk10 MO has a more profound effect on ENS colonization than is observed in the homozygous mapk10Δ10 (compare Figs 4C to 5A). One possible explanation is that non-specific defects caused by MOs, such as cell death and developmental delays, may exacerbate the phenotype [21]. Alternatively, it has recently been suggested that compensatory mechanisms may occur in zebrafish genetic mutants, leading to milder phenotypes in genetic mutants than those generated by MOs [37]. Thus, these observations underscore the utility in employing a range of functional assays in order to validate results.
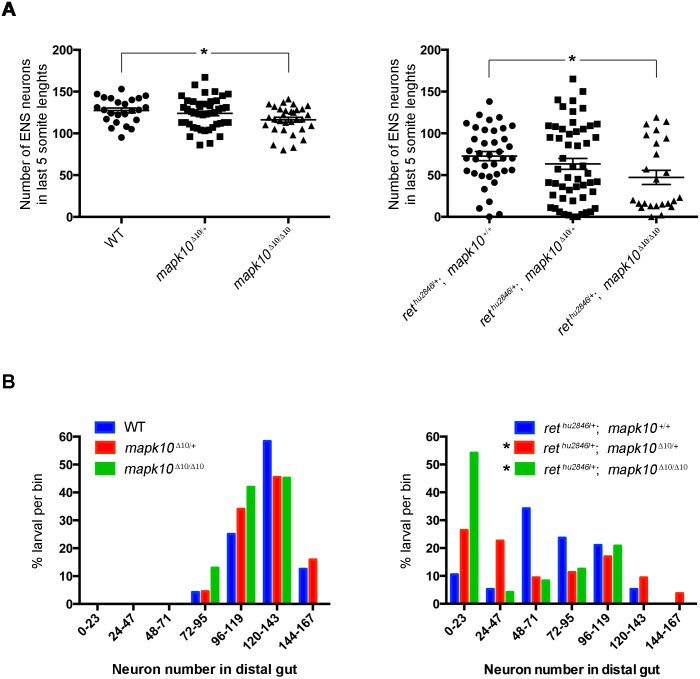
Fig 5. mapk10 as candidate to account for varying expressivity in HSCR.
(A) Enteric neuron number in the distal gut quantified in 4dpf larvae resulting from crosses between mapk10Δ10/+ and rethu2846/+;mapk10Δ10/+ zebrafish. No difference was detected between WT and heterozygous mapk10Δ10 larvae, but mapk10Δ10/Δ10 larvae have a small, but statistically significant reduction in ENS cell number in the gut relative to WT (one-way ANOVA, p = 0.0442, Tukey post-hoc), indicating a role for mapk10 in normal ENS development. No significant difference was detected between rethu2846/+ and mapk10Δ10/+;rethu2846/+ larvae, however, loss of mapk10 in the rethu2846/+ background leads to statistically significant reduction in neuron number (Welch’s one-way ANOVA, p = 0.048). (B) To examine phenotype distribution, individual zebrafish were binned according to neuron number in the distal gut. WT, mapk10Δ10/+ and mapk10Δ10/Δ10 larvae show normal distribution of phenotypes (blue, red, and green bars, respectively, Shapiro-Wilk normality test, for WT: p = 0.776, for mapk10Δ10/+: p = 0.910, and for mapk10Δ10/Δ10: p = 0.1149). Although rethu2846/+ larvae guts are phenotypic, they display a range of neuron numbers in the distal gut region, reflecting normal distribution of mild and progressively more severe colonization phenotypes (Shapiro-Wilk normality test, p = 0.5720, blue bars), counts of neuron number in rethu2846/+;mapk10Δ10/+ (red bars) and rethu2846/+;mapk10Δ10/Δ10 larval guts (green bars) exhibit a non-normal distribution pattern (Shapiro-Wilk normality test, for rethu2846/+;mapk10Δ10/+: p = 0.0054, and for rethu2846/+;mapk10Δ10/Δ10 p = 0.0014), and counts of enteric neuron number in rethu2846/+;mapk10Δ10/Δ10 larvae, showed a statistically significant increase in standard deviation compared with rethu2846/+ larvae (Brown-Forsythe, 0.0445).
Genetic analysis confirms mapk10 as modifier gene for ENS development
To test for genetic interactions between ret and mapk10, we next examined combined effects of mapk10Δ10 and rethu2846 mutations on enteric neuron numbers. No significant difference was detected between rethu2846/+ and mapk10Δ10/+;rethu2846/+ larvae (Fig 5A). In contrast, significant differences were observed in mean neuron number between mapk10Δ10/Δ10;rethu2846/+ and rethu2846/+ larvae (73 neurons in rethu2846/+ vs. 47 neurons in mapk10Δ10/Δ10;rethu2846/+) (Fig 5A). These observations are consistent with effects of mapk10MO and further indicate a role for mapk10 in ENS development.
Interestingly, while the recorded number of neurons in the distal gut of rethu2846/+ larvae showed a normal distribution pattern, the phenotypes of rethu2846/+;mapk10Δ10/+ and rethu2846/+;mapk10Δ10/Δ10 larvae exhibited a non-normal distribution pattern (Fig 5A and 5B). Moreover, counts of enteric neurons in rethu2846/+;mapk10Δ10/Δ10 larvae, showed a statistically significant increase in standard deviation compared with rethu2846/+ larvae (Fig 5A and 5B), with a substantial cluster of severely affected individuals. Specifically, 54% of rethu2846/+;mapk10Δ10/Δ10 larvae had severe phenotypes (less than 24 neurons), relative to only 10% observed amongst rethu2846/+ larvae (Fig 5A and 5B). Taken together these results suggest that loss of mapk10 in ret heterozygous larvae can lead to increased severity of ret-dependent ENS phenotypes, thus highlighting MAPK10 as a candidate gene contributing to expressivity of aganglionosis and manifestation of HSCR.
Discussion
We demonstrate here that Ret signaling is essential for gut innervation of teleosts and employ zebrafish as a model system to gain mechanistic insight into ENS development and the pathogenesis of congenital intestinal neural deficits.
Specifically, we show that the rethu2846 mutant zebrafish line lacks enteric neurons in distal gut segments (the defining feature of HSCR), that individual larvae show varying expressivity of this cellular defect (a well-established feature in the complex genetics of HSCR), and that absence of enteric neurons correlates with defective gut motility (equivalent to the dysmotility associated with the megacolon of HSCR patients). By combining the rethu2846 line with novel transgenic tools and in vivo imaging, we provide evidence that reduced migration of ENCCs is a major contributory factor for distal intestinal aganglionosis. Finally, we demonstrate that this HSCR model can be used as a sensitized background to identify genes that influence ENS phenotype expressivity and therefore are candidate HSCR susceptibility loci. Our current studies focus on mapk10, demonstrating its requirement for development of the full complement of enteric neurons and we show that mutations in this gene can enhance the loss of ENS phenotype of rethu2846 heterozygous larvae.
Decreased ENCC migration speed as major contributor to HSCR-like phenotypes
Assembly of functional intestinal neural circuits depends on overlapping developmental processes, namely survival and proliferation of ENCCs, migration along the gut, and differentiation into multiple neuronal and glial cell types [12, 13]. Although deficits in any of these processes could contribute to intestinal aganglionosis [12, 13], their relative contributions to ENS development remain unclear. By combining the power of zebrafish genetics with the optical accessibility of zebrafish, we demonstrate here that reduced migration speed of ENCC streams is a major determinant of ENS loss in the distal gut of rethu2846/+ larvae. These experiments corroborate recent studies using murine organotypic cultures challenged with pharmacological inhibitors of Ednrb signaling, which concluded that decreased ENCC migratory speed is a principal mechanism of aganglionosis in this paradigm [38]. Since mutations in RET and EDNRB are the most frequent genetic changes encountered in familial cases of HSCR (EDNRB mutations detected in ~5% of HSCR patients) [8], our data, together with those of Young et al. [38], suggest that reduced ENCC migration is a key pathogenic mechanism underlying failure of ganglia formation in the distal colon of HSCR patients. Although we obtained no evidence of cell proliferation defects or apoptotic cell death in rethu2846/+ embryos, potential contributions of these processes in HSCR-like phenotypes remain to be determined. Nevertheless, our current studies highlight differential sensitivity of distinct cellular processes to reduced levels of Ret activity.
Organized motility patterns linked to presence and position of enteric neurons
Although the importance of the ENS in regulating intestinal motility is well established [11], the gut dysmotility observed in mouse models of aganglionosis remains poorly characterized in vivo. By conducting systematic analysis of video recordings of live zebrafish larvae, we demonstrate here that rethu2846 homozygous larvae show dramatic deficits in intestinal motility, highlighted by the almost complete elimination of anterograde peristaltic waves. This finding echoes the loss of intestinal motility observed in sox10colourless homozygous zebrafish lacking enteric neurons [39] and motility changes observed in WT larvae treated with the sodium channel blocker tetrodotoxin (TTX) [30]. Milder defects were observed in rethu2846 heterozygous larvae, and likely mirror the dysmotility observed in HSCR patients [40]. The tractable number of enteric neurons, and the ability to clearly visualize neuronal somata and their projections along the gut enabled us to use individual rethu2846 heterozygous larvae representing different degrees of distal aganglionosis to map with high resolution the endpoints of motility waves relative to the position of the last neuron and/or neural projections. The close association we show between organized peristaltic motility and neuron position builds on previous correlations observed between ganglionic states and motility [41] and makes clear the requirement of local control of muscle by neural networks. The relationship between ganglionated state and capacity to generate motility waves is of clinical interest [42]. Our studies may explain why impaired motility (including constipation) is observed in HSCR patients after transitional zone pull-through (TZPT) (where part of the hypoganglionated gut remains after surgery) [43]. Additionally, our observations argue that the promise of successful treatment of long-segment HSCR by stem cell transplantation [44–47], would require colonization of aganglionic gut segments along their full length. Further work using such zebrafish models has the potential to establish links between states of ganglionosis and their capacity to mediate peristalsis and other ENS regulated processes, and determine, for example, the minimal neurofunctional unit required to restore organized motility. Finally, we suggest that STM analysis of actual intestinal motility can form the basis of highly sensitive assays to assess the role(s) of genetic pathways and environmental (including luminal) factors (i.e. [48] on the assembly of neuronal circuits controlling the motor behavior of the intestine.
rethu2846 heterozygotes as powerful tool in identifying HSCR susceptibility genes
rethu2846/+ larvae reproduce the aganglionosis phenotype of HSCR patients with RET mutations who are invariably heterozygous at this locus [6, 7, 18], and contrasts Ret+/- mice, which show normal development of the ENS [26]. The difference in length and surface area between mouse and human bowel has been cited as a possible reason for phenotypic discrepancy between the two species [7]. However, given the small size of the zebrafish larval gut, our experiments would argue instead that ENS lineages of the mouse are less sensitive to Ret activity levels relative to either human or zebrafish. A potential explanation for this difference is that mouse HSCR-like lines, including those with Ret mutations, are generally maintained on inbred (isogenic) backgrounds. In contrast, mutant zebrafish lines are maintained as outbred populations (due to effects of inbreeding depression) [49, 50], thus sharing with human populations the high degree of genetic heterogeneity associated with outbreeding. Consequently, zebrafish populations are expected to carry greater numbers of individual mutations, with limited genetic pressure to eliminate mutations that would (perhaps even minimally) affect Ret levels. It has been proposed that a full HSCR phenotype results only when >50% of RET function is lost, whether caused by structural or regulatory mutations in RET (as is the case of numerous known coding mutations [8] or the common RET enhancer mutation rs2435357 [51], respectively, or from interactions with other susceptibility genes [52]). Since all rethu2846/+ individual larvae express the same ret loss of function allele, observed variations in expressivity are either due to structural or regulatory mutations at the other ret allele, or mutations at other loci. Therefore, heterozygous rethu2846zebrafish can be used to test the genetic basis of phenotype expressivity.
Human genetic studies and patient genome- or exome- sequencing have identified, in addition to RET, 13 HSCR-associated genes accounting for disease susceptibility [7, 16, 53, 54]. Yet, mutations in these genes account for only ~50% of familial HSCR cases and 20% of sporadic HSCR [51], indicating that novel loci responsible for the missing heritability remain to be uncovered. Here we have used the rethu2846/+ background to provide further evidence that MAPK10 is a HSCR susceptibility locus [34]. By generating a novel mapk10 deletion in zebrafish, we demonstrate that mapk10 is required for normal ENS development in vertebrates and mapk10 mutations dramatically increase severity of ENS phenotypes observed in rethu2846 heterozygous larvae. We hypothesize that in animals in which the influence of mapk10 mutation on phenotypic expressivity is strong, loss of Mapk10 activity may affect, directly or indirectly, Ret signaling. In contrast, in animals in which expressivity is not significantly altered by loss of mapk10, additional factors associated with genetic variation may buffer potential effects of mapk10 deletion. Recent bioinformatics analysis of mouse gene expression profiles in the ENS hypothesize a role for Mapk10 downstream of RET in HSCR [55]. Further study of these interactions, for example in experiments testing the ability of putative downstream factors to rescue of the ret mutant phenotype, can build our understanding of gene regulatory and signaling modules influencing ENS development and impacting on HSCR.
Determining the mechanism by which interacting factors lead to phenotype expressivity will be of significant interest. In the case of mapk10, the exacerbation of ret mutant phenotypes may be caused by causing further delays to progenitor cell migration, by inducing cell death, or reducing ENCC proliferation. It is also possible that a combination of these negative influences leads to the observed phenotypes, since proliferation is itself a force driving ENCC migration [56, 57]. Known functional associations of Jnk/Mapk signaling include roles in differentiation, apoptosis, and migration. It is believed that the diverse roles are due to the large number of Jnk interacting factors [58]. The suggestion from work in mouse is that migration defects is the major consequence of loss of Jnk signaling, since RETS697A mutant mice, which have impaired Mapk signaling (among other signaling defects), exhibit impaired cell migration without alterations in cell proliferation or survival [33]. However, future study of the mapk10 mutant zebrafish will enable a direct examination of the role(s) of Mapk10 in ENS development and in ENS phenotype expressivity.
Because of the multifactorial etiology of HSCR, disease risk cannot be predicted, even in cases of familial HSCR [51, 59]. For this reason genetic counseling is not usually conducted for HSCR, however, surgical strategies are generalized. In the age of personalized medicine, it would be reasonable to expect that if patients could be catalogued based on genetic profiles, then treatment plans, be they surgical or using cell replacement therapies, could be assessed for efficacy amongst different classes of patients. Then, future treatment strategies could be tailored to cope with specific needs of individual patients. To attempt such an approach, the impact of gene mutations on disease expressivity must be understood. Further studies in zebrafish will play a significant role in identifying key regulators of ENS development and their roles in HSCR phenotype expressivity.
Materials and Methods
Animals
All animal experiments were carried out in compliance with the Animals (Scientific Procedures) Act 1986 (UK) and in accordance with the regulatory standards of the UK Home Office (Project Licence 70/7644 to VP). Experiments were also approved by the Animal Welfare and Ethical Review Body (AWERB) of the MRC National Institute for Medical Research/The Francis Crick Institute. Maintenance of zebrafish stocks was performed as described [60]. Embryos and larvae were maintained at 28°C in an illuminated incubator on a 14 h:10 h light:dark cycle. Stages of embryos and larvae were assigned in hours or days post fertilization (hfp or dpf, respectively) according to morphological features [61]. Larvae from 5 to 7dpf were fed ZM-000 (ZM Systems) daily within 30 minutes of onset of light cycle. Tubingen LongFin (TL) was used as background strain for all experiments. In specific experiments (described below), embryos were reared in 0.15M 1-phenyl 2-thiourea (PTU) from 24hpf to inhibit melanization. The rethu2846/+ line and its genotyping has been described previously [23]. The SAGFF234A transgenic fish was identified by a large scale genetic screen using a gene trap construct containing Gal4FF [28, 29], and in the case of SAGFF234A, the Gal4 expressing gene trap construct was integrated within the chrm2a gene on Chromosome 4 encoding the cholinergic receptor, muscarinic 2a. The UAS:GFP transgenic fish was described previously [28]. The mapk10Δ10 line was genotyped as follows: PCR product amplified with primers fwd- ATCTCCACACAGGGTTTTGG and rev- CTGAATGTGAATACTCACCACACT, digested with KpnI, and analyzed by gel electrophoresis to detect KpnI site presence (WT allele, 135 and 65bp bands) or absence (mutant allele, 200bp band).
RNA in situ hybridization and antibody staining
RNA in situ hybridization was performed as described previously using a nadl1.2 probe [24] on PTU treated embryos. Probe for mapk10 was generated by RT-PCR amplification using orthologue-specific [62]primers against NM_001037701.1 (GATTCAGCATCGCAAACTCA and GCCGAAATCCAAAATCTTCA amplify an 837bp fragment corresponding to 5’UTR and 5’cds of mapk10). The product was cloned into pGEMT-Easy and sequence verified, named mapk10-3, linearized with SalI, and transcribed with T7 Polymerase. Immunostaining on embryos and larvae was performed essentially as described [63], except that following overnight fixation in 4% PFA at 4°C, samples were washed 3 times in PBS containing 0.1% TritonX (PBT), equilibrated in water for 1 minute, permeabilized in acetone at -20°C, re-equilibrated in water for 1 minute, and washed in PBT before blocking, incubation with primary antibodies, washing and incubation with secondary antibodies as described [63]. Immunostaining after RNA in situ hybridization was performed after post-fixing processed samples for 20 minutes, washing 5 times in PBT, and then incubating with primary antibodies, with all subsequent steps performed as above. Antibodies used as follows: Hu (mouse, Life Technologies A21272, 1:200), GFP (chick, Abcam ab13970, 1:500), BrdU (rat, Abcam, ab6326, 1:200), 5-HT (rabbit, Sigma, 1:2000) and appropriate Alexa Fluor conjugated secondary antibodies (Molecular Probes).
BrdU incorporation for cell proliferation analysis
Embryos were incubated with BrdU for 30 minutes, essentially as described [64] and processed for immunostaining, as above, except embryos were reared in PTU, and following fixation, dehydrated in methanol, rehydrated, washed in PBT, digested in 10mg/ml proteinase K for 10 minutes, washed in PBT, and post-fixed in 4%PFA for 20 minutes. Embryos were then washed in water, equilibrated in 2N HCl, before denaturing for 1 hour in 2N HCl at room temperature, neutralizing with two 10 minute 0.1M sodium tetraborate washes, and washing in PBT before blocking and immunostaining as above.
TUNEL staining for apoptotic cell death analysis
Apoptotic DNA fragmentation detected using the TUNEL method. Performed after immunostaining, as above, essentially as described [65] and using the ApopTag Red In Situ Apoptosis Detection Kit for direct detection of DNA fragmentation.
Time lapse imaging of ENS progenitor cell migration
Embryos were reared in PTU until 48hfp, lightly anaesthetized (0.15mg/mL Tricaine in fish water), and mounted in embryo arrays [66] embedded in 0.6% low melt temperature agarose (made in 0.5x Danieau’s solution with PTU and Tricaine). Time lapse imaging was performed for 36 hours on a Leica TCS SP5 confocal, with z-stacks collected for each embryo at maximal acquisition speed for the imaging set up (frame interval 374 seconds). Fiji [67, 68] with Trackmate plugin [69] was used to calculate travel distance and formulate migration speed of the migratory wavefront (defined as the position of the most distal GFP+ cell).
ENS neuron counting
Quantification of the number or position of neurons in the distal gut was standardized using tissue landmarks to define gut lengths. In most assays, HuC/D+ ENS neurons were counted, starting from the anal pore and continuing towards the intestinal bulb until 5 or 10 somite lengths had been covered, for 4dpf or 3dpf larvae, respectively. Additionally, the position of the 10th most distal HuC/D+ ENS neuron in 3dpf larvae was scored relative to somite lengths from the anal pore.
Video imaging and analysis of intestinal motility
Embryos were reared without PTU. Following feeding at 5dpf and 6dpf, larvae were transferred to a fresh dish on the evening of the 6th dpf, to enable gut motility to be visualized at 7dpf without luminal contents. Individual larvae were transferred into fish water with anesthetic (0.15mg/mL Tricaine in fish water) and laid, free-floating in lateral view in an agarose trough, and imaged at 2.5 frames per second for 16 minutes with a QICAM-Fast camera using QCapture Pro 6.0 software (Q-Imaging). Analysis of the movies was performed using Igor Pro (WaveMetrics) with custom-written algorithms, allowing movement of the gut wall to be computed and mapped over the imaging period. Spatiotemporal maps were generated and used to determine the presence and direction of contractions and contraction frequency (waves 1000 s-1, travel distance (mm), velocity (average speed over total propagated distance; mm s-1), and interval (s), similar to previous methods [30].
Morpholino oligo injection
All mapk10 sequence information based on Ensembl Zv9 build. The mapk10 splice-blocking morpholino oligo (MO) (GeneTools LLC) mapk10 MO targets the intron3/4-exon4 splice junction (TGCCAAAACCCTGTGTGGAGATAAA), leading to excision of exon 4, confirmed by sequencing. Control embryos were injected with Gene Tools standard control MO (CCTCTTACCTCAGTTACAATTTATA). Efficacy of mapk10 MO was tested by RT-PCR essentially as described [24] with primers from exons 3 and 7 (TGTGAAGATTGCCTTTTGTCA and AATGCAGGTGTTTGATTCCA) to amplify a 500 bp band or a 330bp band if exon 4 excision has occurred. Bands of expected sizes were gel isolated and sequence verified as described [24].
TALEN gene targeting
TALEN design and assembly was performed using Golden Gate TALEN assembly, according to protocols from TAL Effector Nucleotide Targeter 2.0 (Cornell University, http://boglab.plp.iastate.edu) [70, 71] as follows: TALEN target site T CCACCTTCACGGTTCTCAAA cggtaccaaaatctaaa GCCCATTGGTTCTGGGGCTC A (including a Kpn1 site), mapk10 TAL left HD HD NI HD HD NG NG HD NI HD NN NN NG NG HD NG HD NI NI NI, mapk10 TAL right, NN NI NN HD HD HD HD NI NN NI NI HD HD NI NI NG NN NN NN HD. Final plasmids used, pCS2-TALDD and pCS2-TALRR, as described [36]. HRM screening protocols essentially as described [36], using primers fwd: ATTCCACCTTCACGGTTCTC, rev: CTGAATGTGAATACTCACCACACT. High fidelity PCR amplification, cloning and sequencing of the mapk10 target site of mapk10Δ10 founder zebrafish, confirmed a 10bp deletion leading to a frame shift, and loss of a KpnI restriction site. PCR genotyping of targeted allele described above.
Statistics
Statistical analysis was performed using Microsoft Excel (Microsoft) and GraphPad Prism software (GraphPad Software). Data sets were tested for differences using the unpaired t Test (for single comparisons), ANOVA followed by a Bonferroni’s post-hoc test or Tukey post-hoc tests (for multiple comparisons), Fisher’s exact (for proportions), Spearman r (for correlations), or Shapiro-Wilk (for normality). Non-normally distributed data sets were analyzed by Welch’s one way ANOVA. A P value of < 0.05 was deemed significant and in figures graded significance was designated as follows: P > 0.05 (ns = non-significant), P < 0.05 (*), P < 0.005 (**), P < 0.0005 (***), and P < 0.0001 (****). Error bars in graphs represent standard error of the mean (SEM).
Supporting Information
7dpf larvae immunostained with the HuC/D antibody to visualize ENS neurons in WT (A) and rethu2846/+ (B-D) larvae. Asterisks indicate end of the gut tube (anal pore), and arrows denote the position of the most distal HuC/D+ neuron. At 7dpf, WT (ret+/+) larvae exhibit HuC/D+ ENS neurons along the full length of the gut (A). Some rethu2846/+ larvae also show full colonization of the gut by HuC/D+ ENS neurons (B), and are referred to as ‘non-phenotypic’ or rethu2846/+ NP. Other rethu2846/+ larvae show varying extents of distal regions lacking HuC/D+ ENS neurons (C, D), and are referred to as ‘phenotypic’ or rethu2846/+ P. High-resolution images of full gut length was constructed by tiling two overlapping micrographs.
(TIF)
SAGFF234A is a transgenic line with the Gal4 expressing gene trap construct integrated within the chrm2a gene on the chromosome 4 encoding the cholinergic receptor, muscarinic 2a. When crossed to transgenic fish carrying the GFP reporter gene downstream of the Gal4 recognition sequence (UAS:GFP) generates transgenic embryos expressing GFP within the ENS at 4dpf (A, B), in both ENS progenitors at 40hpf (C, arrows) and in virtually all HuC/D+ ENS neurons at 4dpf (D). (E) At 7dpf GFP can be detected in cellular processes of HuC/D+ ENS neurons, distal intestine shown (cellular processes of ENS neurons indicated with arrows). The high-resolution image of the full gut length (in D) was constructed by tiling two overlapping micrographs.
(TIF)
(A-C) Proliferation of ENS progenitor cells is equivalent in 48hpf WT (A) and rethu2846/+ (B) larvae, shown using GFP from SAGFF234A;UAS:GFP to label pixel area of ENS progenitors and BrdU (following 30 minute BrdU pulse) to quantify proliferating cells per 220um (1000 pixels) of the distal migratory wavefront of progenitors (p = 0.9182) (C). The area (in pixels squared) (D) and roundness (4*area/(π*major axis2)) (E) of the migratory wavefront was quantified, and significant increases in both parameters reflect the fact that the rethu2846/+ embryo wavefront is not elongated as is seen in WT (p<0.0001 for both area and roundness). (F-I) TUNEL staining on 42hpf WT (F,G) and rethu2846/+ (H,I) larvae does not show any TUNEL+ apoptotic cells (red, G,I) within the GFP+ migratory stream of ENCCs (F,H). (J-M) Differentiation was assayed in the SAGFF234A;UAS:GFP line by comparing HuC/D expression with GFP expression in 4dpf larvae (J,K) and counting differentiated serotonergic cells (5-HT+) within 5dpf larvae (L,M). While HuC/D+ cells show expected significantly decreased numbers in rethu2846/+ larvae (J, L, p = 0.0096 and p = 0.0045, respectively), the percentage of progenitor cells (GFP+Hu-) cells is not significantly altered (K, p = 0.3785), and the percentage of 5-HT+ cells is also not significantly altered (M, p = 0.2188).
(TIF)
RNA in situ hybridization for mapk10 was conducted in SAGFF234A;UAS:GFP embryos at 54 (A) and 72hpf (D). Immunostaining for GFP identifies the stream of migrating ENCCs (B, both streams shown, E, one stream shown). Merged images indicate that cells containing map10 signal localize to the GFP+ population (C, F arrows denote a number of these cells).
(TIF)
(A) Morpholino oligo (MO) gene knockdown of mapk10. Genomic organization of mapk10 (mapk10-001 from Zv9:CU651624.3:8583743:8720716:1) showing the position of the mapk10 splice blocking MO (mapk10 MO) at the intron3/4-exon4 boundary, which leads to excision of exon 4 (underlined). RT-PCR of injected embryos identifies loss of the 170bp exon4 and reduction of RT-PCR product from 500bp to 330bp, which was confirmed by sequencing. Exon 4 excision leads to a frame shift and early truncation of the protein at amino acid (AA) 25, leading to loss of the protein kinase catalytic domain and all Mapk10/Jnk conserved domains. (B) A novel mapk10 mutant line has been generated by TALEN gene editing. TALEN targeting of exon 4 leads to the generation of a 10 base pair deletion in exon4, which leads to loss of a Kpn1 site, a frame shift, and early truncation of the protein at AA64.
(TIF)
Depth coded Z-projected recording of GFP+ ENCC cells of ret+/+;SAGFF234A;UAS:GFP embryos from 48–56hpf with a frame interval 374 seconds.
(AVI)
Depth coded Z-projected recording of GFP+ ENCC cells of rethu2846/+;SAGFF234A;UAS:GFP embryos from 48–56hpf with a frame interval 374 seconds.
(AVI)
Recording (at 2.5 frames per second) of spontaneous intestinal motility of a WT larvae at 7dpf. The spatiotemporal map constructed from this original movie is shown in Fig 3. Movie is sped up 20 times.
(AVI)
Recording (at 2.5 frames per second) of spontaneous intestinal motility of a rethu2846/+ larvae at 7dpf. The spatiotemporal map constructed from this original movie is shown in Fig 3. Movie is sped up 20 times.
(AVI)
Recording (at 2.5 frames per second) of spontaneous intestinal motility of a rethu2846/hu2846 larvae at 7dpf. The spatiotemporal map constructed from this original movie is shown in Fig 3. Movie is sped up 20 times.
(AVI)
Acknowledgments
We wish to thank Steve Martin, Kaska Koltowska, Megan Addison, Qiling Xu, and Sean Constable for experimental and analytical advice and Malcolm Logan and Pachnis lab members for discussions and advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: Medical Research Council UK http://www.mrc.ac.uk/ and The Francis Crick Institute https://www.crick.ac.uk/ (CW10128, TH VP); Research Foundation Flanders (FWO) (G.0921.15) http://www.fwo.be/ (WB PVB); FWO Postdoctoral Fellowship http://www.fwo.be/ (WB); National Bioresource Project from AMED www.amed.go.jp (KK); JSPS KAKENHI JP16H01651 https://www.jsps.go.jp (KK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chakravarti A. Genomic contributions to Mendelian disease. Genome Res. 2011;21(5):643–4. 10.1101/gr.123554.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGreevy JW, Hakim CH, McIntosh MA, Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis Model Mech. 2015;8(3):195–213. 10.1242/dmm.018424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, et al. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10 Suppl 2:S152–71. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell KJ. The genetics of neurodevelopmental disease. Curr Opin Neurobiol. 2011;21(1):197–203. 10.1016/j.conb.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell KJ, Huang ZJ, Moghaddam B, Sawa A. Following the genes: a framework for animal modeling of psychiatric disorders. BMC Biol. 2011;9:76 10.1186/1741-7007-9-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershon MD. Functional anatomy of the enteric nervous system: A developmental perspective relevant to the pathogenesis of Hirschsprung’s disease In: Holschneider AM, Puri P, editors. Hirschsprung’s Disease and Allied Disorders. The Netherlands: Harwood Academic Publishers; 2008. p. pp. 19–58. [Google Scholar]
- 7.McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. 2013;2(1):113–29. 10.1002/wdev.57 [DOI] [PubMed] [Google Scholar]
- 8.Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45(1):1–14. 10.1136/jmg.2007.053959 [DOI] [PubMed] [Google Scholar]
- 9.Kenny SE, Tam PK, Garcia-Barcelo M. Hirschsprung's disease. Semin Pediatr Surg. 2010;19(3):194–200. 10.1053/j.sempedsurg.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286–94. 10.1038/nrgastro.2012.32 [DOI] [PubMed] [Google Scholar]
- 11.Furness JB. The Enteric Nervous System. Malden, MA, USA: Blackwell Publishing; 2006. [Google Scholar]
- 12.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8(6):466–79. 10.1038/nrn2137 [DOI] [PubMed] [Google Scholar]
- 13.Obermayr F, Hotta R, Enomoto H, Young HM. Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol. 2013;10(1):43–57. 10.1038/nrgastro.2012.234 [DOI] [PubMed] [Google Scholar]
- 14.Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366(1):64–73. 10.1016/j.ydbio.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 15.Avetisyan M, Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest. 2015;125(3):899–907. 10.1172/JCI76307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein AM, Hofstra RM, Burns AJ. Building a brain in the gut: development of the enteric nervous system. Clin Genet. 2013;83(4):307–16. 10.1111/cge.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lake JI, Tusheva OA, Graham BL, Heuckeroth RO. Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis. J Clin Invest. 2013;123(11):4875–87. 10.1172/JCI69781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S, et al. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature. 2005;434(7035):857–63. 10.1038/nature03467 [DOI] [PubMed] [Google Scholar]
- 19.Heanue TA, Shepherd IT, Burns AJ. Enteric nervous system development in avian and zebrafish models. Dev Biol. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd I, Eisen J. Development of the zebrafish enteric nervous system. Methods Cell Biol. 2011;101:143–60. 10.1016/B978-0-12-387036-0.00006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6(1):69–77. 10.1089/zeb.2008.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kettleborough RN, Bruijn E, Eeden F, Cuppen E, Stemple DL. High-throughput target-selected gene inactivation in zebrafish. Methods Cell Biol. 2011;104:121–7. 10.1016/B978-0-12-374814-0.00006-9 [DOI] [PubMed] [Google Scholar]
- 23.Knight RD, Mebus K, d'Angelo A, Yokoya K, Heanue T, Tubingen Screen C, et al. Ret signalling integrates a craniofacial muscle module during development. Development. 2011;138(10):2015–24. 10.1242/dev.061002 [DOI] [PubMed] [Google Scholar]
- 24.Heanue TA, Pachnis V. Ret isoform function and marker gene expression in the enteric nervous system is conserved across diverse vertebrate species. Mech Dev. 2008;125(8):687–99. 10.1016/j.mod.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 25.Shepherd IT, Pietsch J, Elworthy S, Kelsh RN, Raible DW. Roles for GFRalpha1 receptors in zebrafish enteric nervous system development. Development. 2004;131(1):241–9. 10.1242/dev.00912 [DOI] [PubMed] [Google Scholar]
- 26.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367(6461):380–3. 10.1038/367380a0 [DOI] [PubMed] [Google Scholar]
- 27.Brooks AS, Oostra BA, Hofstra RM. Studying the genetics of Hirschsprung's disease: unraveling an oligogenic disorder. Clin Genet. 2005;67(1):6–14. 10.1111/j.1399-0004.2004.00319.x [DOI] [PubMed] [Google Scholar]
- 28.Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci U S A. 2008;105(4):1255–60. 10.1073/pnas.0704963105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawakami K, Abe G, Asada T, Asakawa K, Fukuda R, Ito A, et al. zTrap: zebrafish gene trap and enhancer trap database. BMC Dev Biol. 2010;10:105 10.1186/1471-213X-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmberg A, Olsson C, Hennig GW. TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J Exp Biol. 2007;210(Pt 6):1084–91. 10.1242/jeb.000935 [DOI] [PubMed] [Google Scholar]
- 31.Heanue TA, Pachnis V. Expression profiling the developing mammalian enteric nervous system identifies marker and candidate Hirschsprung disease genes. Proc Natl Acad Sci U S A. 2006;103(18):6919–24. 10.1073/pnas.0602152103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichihara M, Murakumo Y, Takahashi M. RET and neuroendocrine tumors. Cancer Lett. 2004;204(2):197–211. 10.1016/S0304-3835(03)00456-7 [DOI] [PubMed] [Google Scholar]
- 33.Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, et al. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development. 2006;133(22):4507–16. 10.1242/dev.02616 [DOI] [PubMed] [Google Scholar]
- 34.Jiang Q, Ho YY, Hao L, Nichols Berrios C, Chakravarti A. Copy number variants in candidate genes are genetic modifiers of Hirschsprung disease. PLoS One. 2011;6(6):e21219 10.1371/journal.pone.0021219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Y, Clark KJ, Campbell JM, Panetta MR, Guo Y, Ekker SC. Making designer mutants in model organisms. Development. 2014;141(21):4042–54. 10.1242/dev.102186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012;8(8):e1002861 10.1371/journal.pgen.1002861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, et al. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. 2015;524(7564):230–3. 10.1038/nature14580 [DOI] [PubMed] [Google Scholar]
- 38.Young HM, Bergner AJ, Simpson MJ, McKeown SJ, Hao MM, Anderson CR, et al. Colonizing while migrating: how do individual enteric neural crest cells behave? BMC Biol. 2014;12:23 10.1186/1741-7007-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhlman J, Eisen JS. Genetic screen for mutations affecting development and function of the enteric nervous system. Dev Dyn. 2007;236(1):118–27. 10.1002/dvdy.21033 [DOI] [PubMed] [Google Scholar]
- 40.Heuckeroth RO. Hirschsprung Disease In: Faure C, Di Lorenzo C, Thapar N, editors. Pediatric Neurogastroenterology: Gastrointestinal Motility and Functional Disorders in Children. Clinical Gastroenterology. New York, Heidelberg, Dordrecht, London: Springer Science & Business Media; 2012. [Google Scholar]
- 41.Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung's disease. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G996–G1008. 10.1152/ajpgi.00558.2007 [DOI] [PubMed] [Google Scholar]
- 42.Kapur RP. Practical pathology and genetics of Hirschsprung's disease. Semin Pediatr Surg. 2009;18(4):212–23. 10.1053/j.sempedsurg.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 43.Langer JC. Persistent obstructive symptoms after surgery for Hirschsprung's disease: development of a diagnostic and therapeutic algorithm. J Pediatr Surg. 2004;39(10):1458–62. . [DOI] [PubMed] [Google Scholar]
- 44.Burns AJ, Goldstein AM, Newgreen DF, Stamp L, Schafer KH, Metzger M, et al. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev Biol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burns AJ, Thapar N. Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol. 2014;11(5):317–28. 10.1038/nrgastro.2013.226 [DOI] [PubMed] [Google Scholar]
- 46.Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotta R, Cheng LS, Graham HK, Pan W, Nagy N, Belkind-Gerson J, et al. Isogenic enteric neural progenitor cells can replace missing neurons and glia in mice with Hirschsprung disease. Neurogastroenterol Motil. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schill EM, Lake JI, Tusheva OA, Nagy N, Bery SK, Foster L, et al. Ibuprofen slows migration and inhibits bowel colonization by enteric nervous system precursors in zebrafish, chick and mouse. Dev Biol. 2016;409(2):473–88. 10.1016/j.ydbio.2015.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shinya M, Sakai N. Generation of highly homogeneous strains of zebrafish through full sib-pair mating. G3 (Bethesda). 2011;1(5):377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guryev V, Koudijs MJ, Berezikov E, Johnson SL, Plasterk RH, van Eeden FJ, et al. Genetic variation in the zebrafish. Genome Res. 2006;16(4):491–7. 10.1101/gr.4791006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emison ES, Garcia-Barcelo M, Grice EA, Lantieri F, Amiel J, Burzynski G, et al. Differential contributions of rare and common, coding and noncoding Ret mutations to multifactorial Hirschsprung disease liability. Am J Hum Genet. 2010;87(1):60–74. 10.1016/j.ajhg.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Kido T, Garcia-Barcelo MM, Tam PK, Tabatabai ZL, Lau YF. SRY interference of normal regulation of the RET gene suggests a potential role of the Y-chromosome gene in sexual dimorphism in Hirschsprung disease. Hum Mol Genet. 2015;24(3):685–97. 10.1093/hmg/ddu488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alves MM, Sribudiani Y, Brouwer RW, Amiel J, Antinolo G, Borrego S, et al. Contribution of rare and common variants determine complex diseases-Hirschsprung disease as a model. Dev Biol. 2013;382(1):320–9. 10.1016/j.ydbio.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 54.Jiang Q, Arnold S, Heanue T, Kilambi KP, Doan B, Kapoor A, et al. Functional loss of semaphorin 3C and/or semaphorin 3D and their epistatic interaction with ret are critical to Hirschsprung disease liability. Am J Hum Genet. 2015;96(4):581–96. 10.1016/j.ajhg.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schriemer D, Sribudiani Y, A IJ, Natarajan D, MacKenzie KC, Metzger M, et al. Regulators of gene expression in Enteric Neural Crest Cells are putative Hirschsprung disease genes. Dev Biol. 2016. [DOI] [PubMed] [Google Scholar]
- 56.Ridenour DA, McLennan R, Teddy JM, Semerad CL, Haug JS, Kulesa PM. The neural crest cell cycle is related to phases of migration in the head. Development. 2014;141(5):1095–103. 10.1242/dev.098855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol. 2007;302(2):553–68. 10.1016/j.ydbio.2006.10.017 [DOI] [PubMed] [Google Scholar]
- 58.Zeke A, Misheva M, Remenyi A, Bogoyevitch MA. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol Mol Biol Rev. 2016;80(3):793–835. 10.1128/MMBR.00043-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Widowati T, Melhem S, Patria SY, de Graaf BM, Sinke RJ, Viel M, et al. RET and EDNRB mutation screening in patients with Hirschsprung disease: Functional studies and its implications for genetic counseling. Eur J Hum Genet. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westerfield M. The Zebrafish Book. 4th Edition ed Eugene, Oregon: University of Oregon Press; 2000. [Google Scholar]
- 61.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- 62.Seo J, Asaoka Y, Nagai Y, Hirayama J, Yamasaki T, Namae M, et al. Negative regulation of wnt11 expression by Jnk signaling during zebrafish gastrulation. J Cell Biochem. 2010;110(4):1022–37. 10.1002/jcb.22616 [DOI] [PubMed] [Google Scholar]
- 63.Uyttebroek L, Shepherd IT, Harrisson F, Hubens G, Blust R, Timmermans JP, et al. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio). J Comp Neurol. 2010;518(21):4419–38. 10.1002/cne.22464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koltowska K, Apitz H, Stamataki D, Hirst EM, Verkade H, Salecker I, et al. Ssrp1a controls organogenesis by promoting cell cycle progression and RNA synthesis. Development. 2013;140(9):1912–8. 10.1242/dev.093583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132(9):2057–67. 10.1242/dev.01791 [DOI] [PubMed] [Google Scholar]
- 66.Megason SG. In toto imaging of embryogenesis with confocal time-lapse microscopy. Methods Mol Biol. 2009;546:317–32. 10.1007/978-1-60327-977-2_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaqaman K, Loerke D, Mettlen M, Kuwata H, Grinstein S, Schmid SL, et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat Methods. 2008;5(8):695–702. 10.1038/nmeth.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39(12):e82 10.1093/nar/gkr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doyle EL, Hummel AW, Demorest ZL, Starker CG, Voytas DF, Bradley P, et al. TAL effector specificity for base 0 of the DNA target is altered in a complex, effector- and assay-dependent manner by substitutions for the tryptophan in cryptic repeat -1. PLoS One. 2013;8(12):e82120 10.1371/journal.pone.0082120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
7dpf larvae immunostained with the HuC/D antibody to visualize ENS neurons in WT (A) and rethu2846/+ (B-D) larvae. Asterisks indicate end of the gut tube (anal pore), and arrows denote the position of the most distal HuC/D+ neuron. At 7dpf, WT (ret+/+) larvae exhibit HuC/D+ ENS neurons along the full length of the gut (A). Some rethu2846/+ larvae also show full colonization of the gut by HuC/D+ ENS neurons (B), and are referred to as ‘non-phenotypic’ or rethu2846/+ NP. Other rethu2846/+ larvae show varying extents of distal regions lacking HuC/D+ ENS neurons (C, D), and are referred to as ‘phenotypic’ or rethu2846/+ P. High-resolution images of full gut length was constructed by tiling two overlapping micrographs.
(TIF)
SAGFF234A is a transgenic line with the Gal4 expressing gene trap construct integrated within the chrm2a gene on the chromosome 4 encoding the cholinergic receptor, muscarinic 2a. When crossed to transgenic fish carrying the GFP reporter gene downstream of the Gal4 recognition sequence (UAS:GFP) generates transgenic embryos expressing GFP within the ENS at 4dpf (A, B), in both ENS progenitors at 40hpf (C, arrows) and in virtually all HuC/D+ ENS neurons at 4dpf (D). (E) At 7dpf GFP can be detected in cellular processes of HuC/D+ ENS neurons, distal intestine shown (cellular processes of ENS neurons indicated with arrows). The high-resolution image of the full gut length (in D) was constructed by tiling two overlapping micrographs.
(TIF)
(A-C) Proliferation of ENS progenitor cells is equivalent in 48hpf WT (A) and rethu2846/+ (B) larvae, shown using GFP from SAGFF234A;UAS:GFP to label pixel area of ENS progenitors and BrdU (following 30 minute BrdU pulse) to quantify proliferating cells per 220um (1000 pixels) of the distal migratory wavefront of progenitors (p = 0.9182) (C). The area (in pixels squared) (D) and roundness (4*area/(π*major axis2)) (E) of the migratory wavefront was quantified, and significant increases in both parameters reflect the fact that the rethu2846/+ embryo wavefront is not elongated as is seen in WT (p<0.0001 for both area and roundness). (F-I) TUNEL staining on 42hpf WT (F,G) and rethu2846/+ (H,I) larvae does not show any TUNEL+ apoptotic cells (red, G,I) within the GFP+ migratory stream of ENCCs (F,H). (J-M) Differentiation was assayed in the SAGFF234A;UAS:GFP line by comparing HuC/D expression with GFP expression in 4dpf larvae (J,K) and counting differentiated serotonergic cells (5-HT+) within 5dpf larvae (L,M). While HuC/D+ cells show expected significantly decreased numbers in rethu2846/+ larvae (J, L, p = 0.0096 and p = 0.0045, respectively), the percentage of progenitor cells (GFP+Hu-) cells is not significantly altered (K, p = 0.3785), and the percentage of 5-HT+ cells is also not significantly altered (M, p = 0.2188).
(TIF)
RNA in situ hybridization for mapk10 was conducted in SAGFF234A;UAS:GFP embryos at 54 (A) and 72hpf (D). Immunostaining for GFP identifies the stream of migrating ENCCs (B, both streams shown, E, one stream shown). Merged images indicate that cells containing map10 signal localize to the GFP+ population (C, F arrows denote a number of these cells).
(TIF)
(A) Morpholino oligo (MO) gene knockdown of mapk10. Genomic organization of mapk10 (mapk10-001 from Zv9:CU651624.3:8583743:8720716:1) showing the position of the mapk10 splice blocking MO (mapk10 MO) at the intron3/4-exon4 boundary, which leads to excision of exon 4 (underlined). RT-PCR of injected embryos identifies loss of the 170bp exon4 and reduction of RT-PCR product from 500bp to 330bp, which was confirmed by sequencing. Exon 4 excision leads to a frame shift and early truncation of the protein at amino acid (AA) 25, leading to loss of the protein kinase catalytic domain and all Mapk10/Jnk conserved domains. (B) A novel mapk10 mutant line has been generated by TALEN gene editing. TALEN targeting of exon 4 leads to the generation of a 10 base pair deletion in exon4, which leads to loss of a Kpn1 site, a frame shift, and early truncation of the protein at AA64.
(TIF)
Depth coded Z-projected recording of GFP+ ENCC cells of ret+/+;SAGFF234A;UAS:GFP embryos from 48–56hpf with a frame interval 374 seconds.
(AVI)
Depth coded Z-projected recording of GFP+ ENCC cells of rethu2846/+;SAGFF234A;UAS:GFP embryos from 48–56hpf with a frame interval 374 seconds.
(AVI)
Recording (at 2.5 frames per second) of spontaneous intestinal motility of a WT larvae at 7dpf. The spatiotemporal map constructed from this original movie is shown in Fig 3. Movie is sped up 20 times.
(AVI)
Recording (at 2.5 frames per second) of spontaneous intestinal motility of a rethu2846/+ larvae at 7dpf. The spatiotemporal map constructed from this original movie is shown in Fig 3. Movie is sped up 20 times.
(AVI)
Recording (at 2.5 frames per second) of spontaneous intestinal motility of a rethu2846/hu2846 larvae at 7dpf. The spatiotemporal map constructed from this original movie is shown in Fig 3. Movie is sped up 20 times.
(AVI)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.