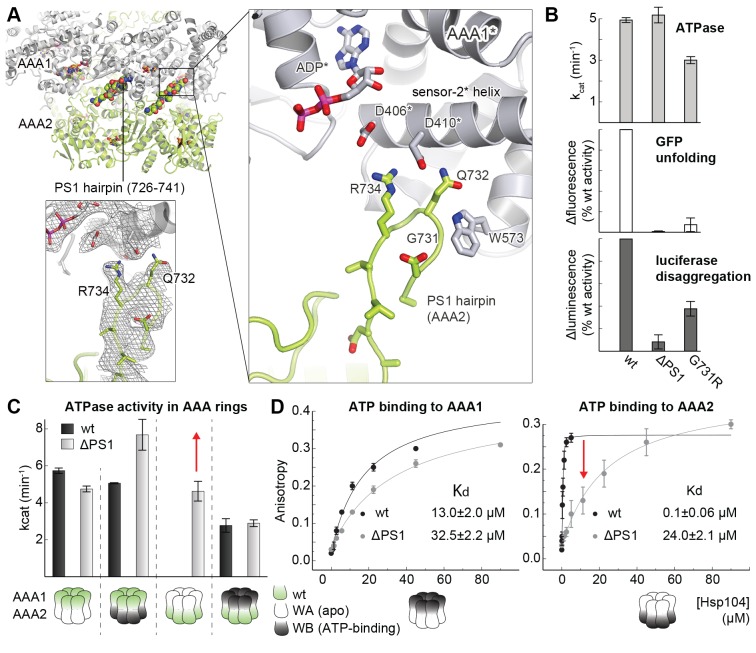
Figure 6. Functional coupling of the two AAA rings of Hsp104.
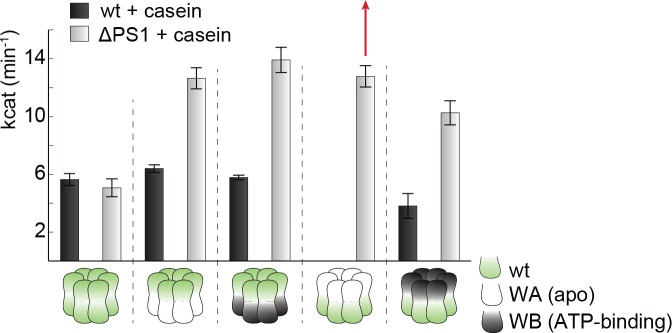
(A) The PS1-hairpin of AAA2, which was well-defined by electron density (inset: omit density map contoured at 1.0 σ), forms specific contacts within the AAA1* active site. Bound ADP and interacting residues are shown in stick mode. (B) Characterization of the PS1 deletion (∆PS1) and the G731R mutant showing that the PS1-hairpin is essential for unfoldase and disaggregase activity, but not for ATPase activity. (C and D) ATPase and mant-ATP binding assays reveal the role of the PS1-hairpin in adjusting the activities and nucleotide binding affinities of AAA1 and AAA2 to each other. Strongest effects of the ∆PS1 mutation are highlighted (red arrow). The used AAA variants (WA/WB combined with wildtype) are indicated. Error bars represent standard deviations.
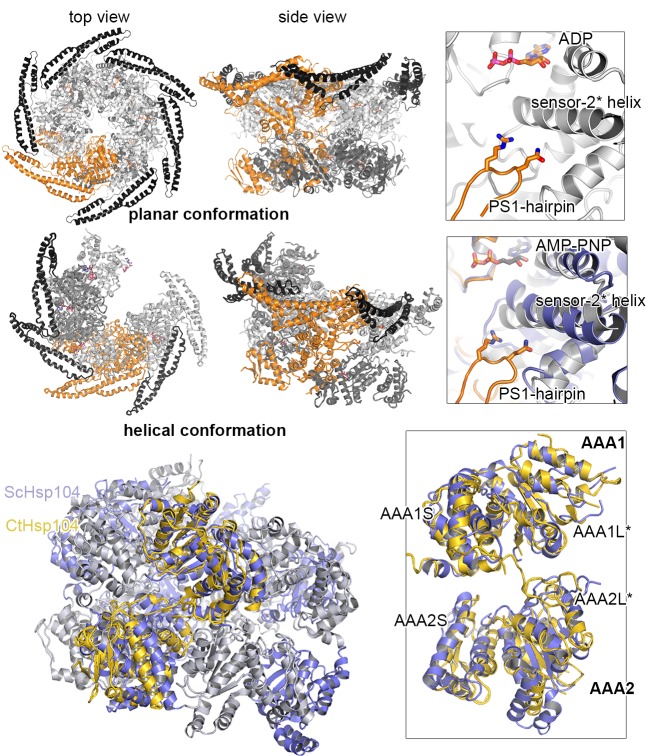
Figure 6—figure supplement 1. Position of the PS1-hairpin in hexameric Hsp104.
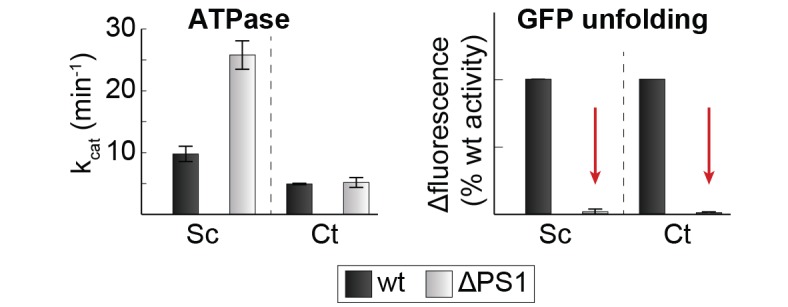
Figure 6—figure supplement 2. Effect of PS1-hairpin deletion on ScHsp104 activity.
Figure 6—figure supplement 3. SEC profiles of CtHsp104 dWA and dWB mutants.