This is the first study to demonstrate improved endothelial function after an acute bout of resistance-based interval exercise. Our data indicate a potential therapeutic effect of resistance interval exercise on endothelial function in older adults with and without type 2 diabetes. The mechanisms underlying these effects warrant further investigation.
Keywords: flow-mediated dilation, blood pressure, vascular function, blood flow, strength training, high-intensity interval exercise, high-intensity interval training, HIIT
Abstract
Different modes of exercise, disease, and training status can modify endothelial shear stress and result in distinct effects on endothelial function. To date, no study has examined the influence of type 2 diabetes (T2D) and training status on the acute endothelial response to different modes of interval exercise (INT). We examined the effect of a single session of resistance- and cardio-based INT compared with a time-matched control on endothelial function in 12 age-matched T2D participants, 12 untrained, and 11 trained adults (aged 56 ± 7 yr). Flow-mediated dilation (%FMD) of the brachial artery was assessed at baseline and immediately, 1, and 2 h after an acute bout of cardio interval (C-INT), resistance interval (R-INT), and seated control (CTL); these interventions were randomized and separated by >2 days. C-INT involved seven 1-min cycling intervals at 85% of peak power with 1-min recovery between. R-INT involved the same pattern of seven 1-min intervals using leg resistance exercises. Endothelial function (%FMD) was improved after R-INT in all groups (Condition × Time interaction, P < 0.01), an effect that was most robust in T2D where %FMD was higher immediately (+4.0 ± 2.8%), 1 h (+2.5 ± 2.5%), and 2 h (+1.9 ± 1.9%) after R-INT compared with CTL (P < 0.01 for all). C-INT improved %FMD in T2D at 1-h postexercise (+1.6 ± 2.2%, P = 0.03) compared with CTL. In conclusion, R-INT acutely improves endothelial function throughout the 2-h postexercise period in T2D patients. The long-term impact of resistance exercise performed in an interval pattern is warranted.
NEW & NOTEWORTHY
This is the first study to demonstrate improved endothelial function after an acute bout of resistance-based interval exercise. Our data indicate a potential therapeutic effect of resistance interval exercise on endothelial function in older adults with and without type 2 diabetes. The mechanisms underlying these effects warrant further investigation.
the benefits of regular exercise are far more pervasive than the effect on traditional cardiovascular risk factors alone; improvements in endothelial function may explain a large proportion of the risk reduction (27). The endothelium plays a pivotal role regulating the many factors that determine vascular tone, tissue perfusion, coagulation, and inflammation (12). Endothelial dysfunction is an early manifestation in many chronic diseases, including diabetes (20), and contributes to the approximately two- to fourfold greater risk of cardiovascular disease in type 2 diabetes (T2D) (20). Exercise interventions involving aerobic and resistance exercise can improve endothelial function (29, 37), a response largely mediated by acute elevations in blood flow and laminar shear stress during individual exercise bouts (41). The effect of an acute bout of cardio- or resistance-based exercise, performed in an interval pattern, on the endothelium of adults with T2D has not been investigated. It is known that different exercise modes and intensities modify the shear stress stimulus and may result in distinct responses in endothelial function (38, 41) but the impact of exercise mode, in addition to T2D or training status, is unclear.
There is continued widespread interest in interval exercise (INT) because it has been shown to improve cardiometabolic health with relatively minimal time commitment (4, 16, 46). INT alternates high and low intensity exercise periods, often in a 1:1 work:rest ratio (14, 46). This pattern of exercise may be attractive and makes vigorous exercise attainable for most individuals because it incorporates built in rest/recovery periods (14). A single session of INT has been shown to improve endothelial function in coronary artery disease patients (aged ∼66 yr) (10) and lower 24-h glucose in T2D (15). Resistance exercise may be more effective than cardio for improving vascular function and remodeling (35, 37, 44), although this is not a universal finding (32). Resistance and cardio exercise can be effectively performed as INT; for example, in insulin-resistant individuals combined resistance- and cardio-based interval exercise was just as effective as cardio-based INT for improving glucose control (13). It is possible that the addition of resistance exercise to the oscillatory pattern of high- and low-intensity INT exercise may offer a prophylactic effect on the vasculature (47). Despite this, no study has investigated the effects of leg resistance INT alone and most of the literature has investigated the endothelial responses after cardio-based continuous exercise (reviewed in Ref. 11).
In addition to exercise parameters, inconsistent findings surrounding acute exercise and endothelial function (reviewed in Ref. 11) may be due to vascular risk factors (e.g., T2D) and/or training status. For example, Hallmark et al. (21) found that while high-intensity exercise improved endothelial function in lean adults, there was no effect in obese adults (21). Similarly, in inactive overweight men endothelial function was decreased after exercise, independent of exercise intensity, compared with an increase in active overweight men (22). These studies suggest that the presence of vascular risk factors and/or habitual activity levels may modulate the impact of acute exercise on endothelial function.
Given the clinical and functional importance of changes in endothelial function, we sought to examine the effect of two common exercise modes performed as INT in age matched T2D, untrained, and highly trained normoglycemic adults. The primary purpose was to examine the effects of cardio- and resistance-INT on endothelial function measured by flow-mediated dilation. The secondary aim was to examine the influence of INT mode on shear stress, blood flow, and blood pressure. We tested the hypothesis that both acute cardio- and resistance-INT would lead to improvements in endothelial function compared with a time-matched control.
METHODS
Study Overview and Prescreening
A randomized crossover design was used to compare the vascular response to cardio-INT (C-INT) and resistance-INT (R-INT) relative to a time-matched control condition (CTL) in age-matched T2D, normoglycemic adults who met current physical activity guidelines but were not participating in a structured exercise training program (UN-NG) and highly-trained normoglycemic adults (TR-NG). The study protocol was approved by the University of British Columbia Clinical Research Ethics Board and all participants provided written informed consent. Before participation T2D participants were screened using a 12-lead ECG exercise stress test and cleared for vigorous exercise by a cardiologist. All participants then completed a maximal exercise test on a cycle ergometer to determine cardiorespiratory fitness (V̇o2 peak). The T2D patients had been familiarized with six sessions of exercise [2 R-INT and 4 C-INT sessions involving 4–6 × 1-min intervals at a rating of perceived exertion (RPE) corresponding to ∼5 on the CR-10 scale (6)] across 2 wk to introduce them to INT and build up to the exercise protocols for testing days. Baseline investigations were performed after 48 h of rest from a previous exercise session to avoid the acute effects of exercise on baseline values. UN-NG and TR-NG maintained their typical physical activity habits throughout the study but similar to T2D participants refrained from exercise for 48 h before testing sessions. UN-NG and TR-NG were screened using a Physical Activity Readiness Questionnaire-Plus (PAR-Q+) and a health-screening questionnaire that included a Godin Leisure Time Physical Activity Questionnaire. TR-NG were defined by completing >7 h of endurance training per week and were in the >80th percentile for age- and gender-adjusted V̇o2 peak based on data from the NHANES and Aerobics Centre Longitudinal Study (5, 7, 31) (range 37–63 ml·kg−1·min−1). UN-NG self-reported performing 213 ± 145 min/wk of light and/or 115 ± 145 min/wk of moderate physical activity (42) and had a V̇o2 peak in the 20-50th percentile (range 20–35 ml·kg−1·min−1).
Participants
Thirty-five participants (40% male, 60% female, average age 56 ± 7 yr, range 40–66 yr) volunteered to participate and completed two initial and three experimental testing sessions. Baseline characteristics of participants in the three groups are shown in Table 1. All participants were nonsmoking and were instructed to replicate any vitamin or supplement intake exactly before each experimental session (verified by food records and interviews). T2D participants were on stable medications and were physician diagnosed for at least 6 mo (range 2–17 yr) before the study, and they were well controlled (HbA1c <8.0%) and not on exogenous insulin. In addition, exclusion criteria included diagnosed diabetic neuropathy, chronic kidney disease, heart and coronary artery disease, and any other contraindication to vigorous exercise. T2D participants on oral hypoglycemic medications followed normal prescriptions, which were replicated exactly for all experimental sessions. Diabetes medications included metformin only (n = 9), DPP4 inhibitor only (n = 1), SGLT2 inhibitor + GLP-1 agonist (n = 1), and sulfonylurea + GLP-1 agonist (n = 1). Hypertensive medications included ACE inhibitor (n = 7), angiotensin receptor blocker (n = 2), and calcium channel blocker (n = 1). All non-T2D participants were free from any diagnosed chronic disease and not taking medications, except one participant in the UN-NG group who was taking 5 mg of felodipine (calcium channel blocker) daily for hereditary elevated blood pressure. All females were postmenopausal (no menstruation for >12 mo), except for two females in the TR-NG group.
Table 1.
Baseline characteristics of type 2 diabetes, untrained normoglycemic, and trained normoglycemic adults
| T2D | UN-NG | TR-NG | |
|---|---|---|---|
| n = | 12 (6 males) | 12 (6 males) | 11 (7 males) |
| Age, yr | 57.5 ± 5.0 | 55.3 ± 9.1 | 55.1 ± 7.0 |
| BMI, kg/m2 | 35 ± 7 | 26 ± 5 | 23 ± 3* |
| Body fat, % | 32.4 ± 7.5 | 23.9 ± 4.2* | 15.8 ± 5.9*† |
| V̇o2peak, ml·kg−1·min−1 | 19 ± 4† | 29 ± 6* | 45 ± 7*† |
| HRpeak, beats/min | 161 ± 12 | 160 ± 20 | 170 ± 9 |
Values are mean ± SD.
T2D, type 2 diabetic adults; UN-NG, untrained normoglycemic adults; TR-NG, trained normoglycemic adults; BMI, body mass index; HRpeak, maximal heart rate; V̇o2peak, cardiorespiratory fitness.
P < 0.05 vs. T2D.
P < 0.05 vs. UN-NG.
Experimental Protocol
Pretesting.
Height and weight were measured using a stadiometer and balance beam scale (Seca 700, Hamburg, Deutschland) and body composition assessed by DXA (Hologic Discovery DXA; Fig. 1). A maximal incremental exercise test (increasing 1 W every 4 s) to volitional exhaustion was performed on an electronically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) to determine maximal oxygen uptake (V̇o2 peak), heart rate (HRpeak), and power output (Wpeak). The test began at 30 W for T2D and UN-NG participants and 100 W for TR-NG participants.
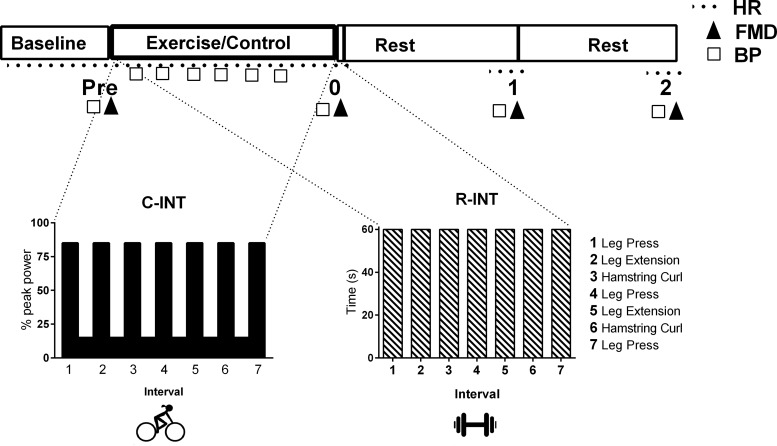
Fig. 1.
Schematic illustrating the timeline of the experimental trials, including a figure illustrating the cardio-based (C-INT) and resistance-based (R-INT) interval exercise protocols, which were performed in a random order with a sitting-control condition (CTL). Flow-mediated dilation (FMD) and blood pressure (BP) were measured before (Pre), immediately (0), 1, and 2 h after each experimental trial. HR, heart rate.
Experimental trials.
Participants completed three, 3-h experimental trials in a randomized order with at least 48 h recovery between (Fig. 1). Exercise was controlled for 48 h before each trial, which began at either 1100 or 1600 (same time within participants) 4 h after consumption of a standardized meal. No food or drink other than water was consumed throughout the trial. Physiological measures were taken at baseline, immediately (within 5 min), 1 h, and 2 h after exercise/sitting-control. Between measurements participants remained in the laboratory in a resting seated position. Baseline measurements for each experimental trial were taken after 15 min of supine rest. All measurements were performed in a temperature-controlled, quiet, and dimly lit room.
Cardio-based interval exercise.
All participants completed seven 1-min intervals on the aforementioned cycle ergometer at 85% Wpeak, alternated with 1-min recovery at 15% Wpeak (Fig. 1). Participants were instructed to increase their cadence to between 80 and 100 revolutions per min (rpm) during the vigorous intervals. Heart rate (continuous 12-lead ECG), manual blood pressure (obtained in last 30 s of alternate work and rest intervals), and RPE (6) were recorded at the end of each interval.
Resistance-based interval exercise.
All participants completed seven 1-min intervals of leg resistance exercise with 1-min recovery, with matched duration, pattern, and muscle groups as C-INT (Fig. 1). Familiarization for the three leg resistance exercises involved one set of six to eight repetitions using a weight selected out of three levels consisting of 5 lb increments. The participants were asked if they could complete this exercise for 1-min based on an RPE of ∼5 (“hard”) such that they were able complete each 1-min interval. For each 1-min “hard” interval participants completed as many reps as possible of each exercise, alternated with 1-min recovery where participants walked to the next exercise station. Resistance level, repetitions, heart rate, blood pressure, and RPE were recorded for each interval. This R-INT protocol was designed to target the same major muscle groups in a similar 1-min on:off pattern as C-INT, while eliciting a similar RPE (Table 2). Blood pressure (manual BP in last 30-s of each 1-min interval), heart rate (Polar H1, Kempele, Finland), and RPE were recorded in the last 10-s of each interval. Both exercise protocols began with a 3 min warm-up and ended with a 3-min cool-down performed on a cycle ergometer at a self-selected pace (rpm) at 30–50 W.
Table 2.
Blood pressure, heart rate, and RPE during the intervals for cardio and resistance interval exercise for T2D, UN-NG, and TR-NG participants
| C-INT |
R-INT |
|||||
|---|---|---|---|---|---|---|
| T2D | UN-NG | TR-NG | T2D | UN-NG | TR-NG | |
| RPE | 5 ± 1 | 5 ± 2 | 5 ± 1 | 5 ± 2 | 5 ± 2 | 5 ± 1 |
| %HRpeak | 88 ± 6† | 90 ± 6† | 87 ± 6† | 67 ± 7 | 70 ± 10 | 64 ± 8 |
| Systolic blood pressure, mmHg | 192 ± 15 | 177 ± 18 | 174 ± 16 | 196 ± 18 | 178 ± 25 | 191 ± 24 |
| Diastolic blood pressure, mmHg | 87 ± 6 | 79 ± 3* | 77 ± 8* | 95 ± 6† | 87 ± 6† | 90 ± 6† |
Values are mean ± SD.
CTL, control; C-INT, cardio-based interval exercise; R-INT, resistance-based interval exercise; RPE, rate of perceived exertion.
P < 0.05 vs. T2D.
P < 0.05 vs. C-INT.
Control condition.
In the control condition participants sat upright for 20 min in place of the exercise time. Everything else including activity between the measurements and the timing thereof was the same as the exercise trials (Fig. 1).
Physiological Measures
Flow-mediated dilation.
Brachial artery FMD was examined as an index of endothelial function using high-resolution ultrasound (Terason 3200) as per published guidelines (9, 39). Briefly, the right arm of each participant was extended 80° from the torso and a longitudinal image of the artery was obtained 2–3 cm from the antecubital fossa. A rapid inflation and deflation cuff was positioned on the forearm 1–2 cm distal from the olecranon process. Once the image was optimized in B-mode, simultaneous B-mode image and Doppler velocity measurements (insonation angle maintained at 60°) were obtained. Ultrasound data were recorded for a 1-min baseline, 30 s before cuff deflation and continued for 3 min thereafter. The cuff was inflated to >60 mmHg above systolic blood pressure for 5-min to induce forearm ischemia and the subsequent hyperemic stimulus. Probe placement and ultrasound settings were maintained for each participant across each experimental trial. Heart rate (single-lead ECG) and brachial blood pressure (manual sphygmomanometer) were measured before each FMD measurement (Fig. 1). Mean arterial blood pressure (MAP) was calculated as 1/3 × systolic blood pressure (SBP) + 2/3 × diastolic blood pressure (DBP).
Brachial artery diameter and blood flow analysis.
Analyses of brachial artery diameter and blood velocity measures were performed using edge detection software, which reduces user bias and increases accuracy (19, 48). Blood flow (ml/min) was calculated from the product of cross-sectional area and Doppler velocity [velocity × π × (diameter2/4) × 60] and shear rate (s−1) was calculated as (four times velocity/diameter) from synchronized diameter and velocity recordings (19). The shear rate area under the curve (SRAUC) for the hyperemic stimulus was calculated from simultaneous diameter and velocity data from cuff release to peak arterial dilation. Baseline antegrade and retrograde shear rates (s−1) were calculated from antegrade and retrograde mean blood velocities (four times mean baseline antegrade or retrograde velocity ÷ mean baseline diameter). Vascular conductance (ml·min−1·mmHg−1) was calculated as the ratio of mean blood flow to mean arterial pressure. The coefficients of variation of brachial artery diameter and %FMD were 2.1% and 7.3%, respectively, based on baseline measurements preexercise between experimental trials.
FMD is expressed as the absolute change in artery diameter (absolute FMD = postocclusionpeak diameter − preocclusionmean diameter), the percent change in artery diameter from baseline [%FMD = 100 × (absolute FMD/preocclusionmean diameter)], and to adjust for the potential confounder of baseline diameter (Dbase) allometric scaling was used (Dbase-adjusted FMD) (2, 39).
Statistics
Statistical analyses were performed using SPSS 22.0 (SPSS, Chicago, IL). One-way ANOVA was used to examine baseline differences between groups. A three-factor (Group × Condition × Time) ANOVA with repeated measures on condition and time were used to assess significant differences between groups and conditions across time. Post hoc analyses with Bonferonni corrections were used to evaluate significant interactions and main effects (using P < 0.05). Specifically, significant Group × Condition × Time interactions or Condition × Time interactions were probed for differences within groups between R-INT and C-INT, relative to CTL, at each time point. All data were first tested for normality and are reported as mean ± SD. For the primary outcome of %FMD, and for MAP, magnitude-based inference analyses were performed according to contemporary views on statistical reporting, allowing for clinically meaningful inference (3). For this, the spreadsheet for confidence limits and inferences was downloaded from www.newstats.org. The smallest clinically beneficial threshold for %FMD was +1%, based on a recent meta-analyses, which showed a 13% reduced risk of future cardiovascular events for every 1% improvement in %FMD [95% confidence interval (CI): 9 to 17%] (23). In line with previous studies, a 2-mmHg reduction in MAP was considered to be the smallest clinical threshold change for blood pressure (8).
RESULTS
Characteristics of C-INT and R-INT Exercise Sessions
Participants successfully completed both the C-INT and R-INT protocols with no reports of discomfort or excessive changes in blood pressure. All participants completed seven 1-min intervals; however, for C-INT two T2D participants and one UN-NG participant reduced their workload by 10 W for the final two or three 1-min intervals because their RPE was >8 and heart rate was >95% of maximum. Analyses performed with and without the two non-postmenopausal women were not significantly different and did not change the interpretation of the results. Peak heart rate during the C-INT intervals was higher than R-INT (P = 0.01), with no difference between groups (Table 2). Diastolic blood pressure was significantly higher during R-INT compared with C-INT (P < 0.01) and in T2D participants compared with UN-NG and TR-NG (P < 0.01, Table 2). Systolic blood pressure did not significantly differ between C-INT and R-INT exercise protocols or between groups (Table 2).
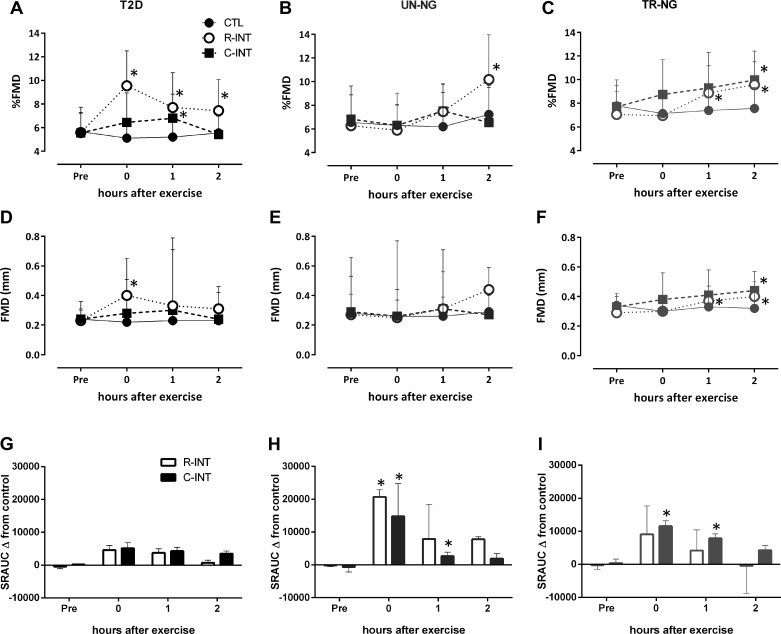
Brachial Artery %FMD
There was a significant Group × Condition × Time interaction for %FMD (Fig. 2, P < 0.01). No change in %FMD was seen across time in CTL nor was it significantly different at baseline between trials within individuals. TR-NG had a higher baseline %FMD (average of three premeasures) than UN-NG (7.8 ± 2.2 vs. 6.6 ± 2.3%, P = 0.03) and T2D (5.7 ± 1.6%, P = 0.01), with no difference between T2D and UN-NG (P = 0.32). When adjusted for baseline diameter using allometric scaling (Dbase-adjusted FMD), there was a significant difference between groups at baseline (TR-NG: 7.7 ± 2.2% vs. UN-NG: 6.6 ± 2.5% vs. T2D: 5.3 ± 1.4%, all P < 0.05).
Fig. 2.
Flow-mediated dilation (%FMD), absolute FMD (mm), and shear rate area under the curve (SRAUC) before, immediately, 1, and 2 h (mean ± SD) after control (CTL), resistance interval exercise (R-INT), and cardio interval exercise C-INT in type 2 diabetes (T2D: A, D, and G), age-matched untrained normoglycemic (UN-NG: B, E, and H), and highly trained normoglycemic (TR-NG: C, F, and I) participants. *P < 0.05, compared with CTL.
T2D.
Post hoc and inferential analyses indicated that in T2D %FMD was significantly higher immediately (95% CI: 3.0 to 5.9%), 1 h (CI: 0.8 to 4.2%), and 2 h (CI: 0.7 to 3.1%) after R-INT compared with CTL; the probability that these effects were most likely beneficial/negligible/harmful were 100/0/0, 96/4/0, and 94/6/0%, respectively. After C-INT compared with CTL, %FMD in T2D was unchanged immediately (CI: −0.5 to 3.1%), higher at 1 h (CI: 0.2 to 3.0%), and unchanged 2 h (CI: −4.5 to 4.3%) following exercise; probability of beneficial/negligible/harmful was 64/35/1, 81/19/0, and 30/37/33%, respectively.
UN-NG.
%FMD after R-INT in UN-NG was unchanged immediately (CI: −5.1 to 4.5%) and 1 h (CI: 0.3 to 2.8%) and higher 2 h following exercise (CI: 0.38 to 5.5%) compared with CTL; probability of beneficial/negligible/harmful was 28/34/38, 64/35/0.4, and 94/6.0/0.3%, respectively. After C-INT compared with CTL %FMD in UN-NG was unchanged immediately (CI: −0.08 to 0.10%), 1 h (CI: −0.6 to 3.2%), and 2 h (CI: −0.06 to 0.02%) following exercise; probability of beneficial/negligible/harmful was 0/100/0, 63/36/1, and 0/100/0%, respectively.
TR-NG.
%FMD after R-INT in TR-NG was unchanged immediately (CI: −0.48 to 0.12%) but higher 1 h (CI: 0.36 to 2.0%) and 2 h following (CI: 1.2 to 2.8%) compared with CTL; probability of beneficial/negligible/harmful was 0/100/0, 68/32/0, and 99/1/0%, respectively. After C-INT compared with CTL %FMD in TR-NG was unchanged immediately (CI: −0.3 to 3.6%) and 1 h (CI: −0.4 to 3.6%) and higher 2 h (CI: 1.4 to 3.4%) following exercise; probability of beneficial/negligible/harmful was 74/25/1, 74/25/1, and 99/1/0%, respectively.
Absolute FMD, Dbase-Adjusted FMD, and Shear Rate AUC
There was a Condition × Group interaction (Fig. 2, P = 0.05) for absolute FMD (mm). Post hoc analyses indicated that in T2D absolute FMD was higher immediately after R-INT compared with CTL (P = 0.03). In TR-NG participants absolute FMD was higher 1 h (P = 0.02) and 2 h (P = 0.01) following R-INT compared with CTL and higher 2 h (P = 0.01) after C-INT compared with CTL. There was no change in absolute FMD in UN-NG participants (Fig. 2). There was a significant Group × Condition interaction for Dbase-adjusted FMD (Table 3, P = 0.03). In T2D Dbase-adjusted FMD was higher immediately (P = 0.05) and 1 h (P = 0.01) after R-INT compared with CTL and higher 1 h (P = 0.01) after R-INT compared with C-INT. In UN-NG and TR-NG participants there were no significant differences for R-INT compared with CTL, or C-INT compared with CTL, for Dbase-adjusted FMD at any time point (Table 3). In UN-NG Dbase-adjusted FMD was higher after R-INT than C-INT immediately postexercise (P = 0.05). Time to peak diameter was not significantly different between conditions or groups (data not shown).
Table 3.
Flow-mediated dilation and hemodynamic responses across time during the sitting-control, acute cardio-based, and resistance-based interval conditions in T2D and age-matched UN-NG and TR-NG
| CTL |
C-INT |
R-INT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Immed-ex | 1 h | 2 h | Baseline | Immed-ex | 1 h | 2 h | Baseline | Immed-ex | 1 h | 2 h | |
| T2D | ||||||||||||
| Baseline diameter, mm | 4.3 ± 0.9 | 4.3 ± 0.9 | 4.3 ± 1.0 | 4.3 ± 0.9 | 4.4 ± 0.9 | 4.4 ± 1.0 | 4.4 ± 1.0 | 4.3 ± 1.0 | 4.3 ± 0.8 | 4.2 ± 0.9 | 4.3 ± 0.9 | 4.2 ± 1.0 |
| Peak diameter, mm | 4.5 ± 0.9 | 4.6 ± 1.0 | 4.6 ± 1.0 | 4.5 ± 0.9 | 4.6 ± 0.9 | 4.7 ± 1.0 | 4.7 ± 1.0 | 4.5 ± 1.0 | 4.5 ± 0.8 | 4.6 ± 1.0 | 4.6 ± 1.0 | 4.5 ± 1.0 |
| Dbase-adjusted FMD | 5.7 ± 1.6 | 5.1 ± 1.6 | 5.2 ± 1.3 | 5.6 ± 1.4 | 6.0 ± 2.2 | 7.1 ± 5.6 | 4.7 ± 6.2 | 6.8 ± 3.1 | 5.0 ± 1.6 | 8.6 ± 5.8* | 9.9 ± 9.1*† | 5.8 ± 5.1 |
| Blood flow, ml/min | 117 ± 55 | 109 ± 47 | 124 ± 65 | 117 ± 58 | 93 ± 26 | 148 ± 61* | 130 ± 23 | 74 ± 28 | 93 ± 38 | 130 ± 62* | 96 ± 36 | 91 ± 42 |
| Systolic BP, mmHg | 124 ± 11 | 126 ± 12 | 128 ± 12 | 127 ± 11 | 128 ± 13 | 124 ± 21* | 124 ± 20* | 124 ± 11* | 125 ± 12 | 125 ± 12 | 123 ± 9* | 122 ± 7* |
| Diastolic BP, mmHg | 79 ± 8 | 81 ± 6 | 80 ± 7 | 80 ± 5 | 77 ± 8 | 76 ± 8 | 79 ± 6 | 79 ± 6 | 79 ± 9 | 78 ± 5 | 77 ± 5 | 78 ± 4 |
| UN-NG | ||||||||||||
| Baseline diameter, mm | 4.2 ± 0.8 | 4.1 ± 0.8 | 4.1 ± 0.7 | 4.1 ± 0.9 | 4.3 ± 1.0 | 4.1 ± 0.9 | 4.2 ± 1.0 | 4.1 ± 0.8 | 4.4 ± 0.8 | 4.4 ± 0.8 | 4.4 ± 0.9 | 4.4 ± 0.9 |
| Peak diameter, mm | 4.5 ± 0.9 | 4.4 ± 0.9 | 4.4 ± 0.8 | 4.3 ± 0.9 | 4.6 ± 0.9 | 4.4 ± 0.9 | 4.5 ± 0.9 | 4.4 ± 0.9 | 4.5 ± 1.2 | 4.5 ± 1.0 | 4.6 ± 1.1 | 4.7 ± 0.9 |
| Dbase-adjusted FMD | 6.5 ± 2.0 | 6.8 ± 3.1 | 6.2 ± 3.0 | 7.1 ± 2.2 | 6.0 ± 4.1 | 6.3 ± 3.6 | 6.0 ± 4.9 | 6.5 ± 3.1 | 7.5 ± 5.1 | 9.6 ± 6.2† | 8.9 ± 5.1 | 8.8 ± 6.5 |
| Blood flow, ml/min | 102 ± 53 | 104 ± 66 | 94 ± 41 | 93 ± 56 | 134 ± 95 | 141 ± 76 | 160 ± 73* | 138 ± 102* | 100 ± 58 | 122 ± 44 | 134 ± 72 | 142 ± 83 |
| Systolic BP, mmHg | 122 ± 12 | 124 ± 12 | 125 ± 13 | 125 ± 12 | 123 ± 10 | 118 ± 15 | 120 ± 11* | 122 ± 13 | 123 ± 14 | 123 ± 15 | 119 ± 12* | 122 ± 16 |
| Diastolic BP, mmHg | 81 ± 7 | 81 ± 6 | 83 ± 7 | 82 ± 6 | 79 ± 5 | 74 ± 9* | 76 ± 7* | 76 ± 7* | 79 ± 8 | 79 ± 6 | 78 ± 5 | 79 ± 8 |
| TR-NG | ||||||||||||
| Baseline diameter, mm | 4.4 ± 0.8 | 4.3 ± 0.8 | 4.4 ± 0.9 | 4.3 ± 0.8 | 4.2 ± 0.4 | 4.3 ± 0.9 | 4.4 ± 0.8 | 4.3 ± 0.8 | 4.4 ± 0.8 | 4.4 ± 0.8 | 4.4 ± 0.9 | 4.4 ± 0.9 |
| Peak diameter, mm | 4.7 ± 0.8 | 4.6 ± 1.0 | 4.7 ± 0.9 | 4.7 ± 0.9 | 4.7 ± 0.8 | 4.8 ± 0.9 | 4.8 ± 0.9 | 4.8 ± 1.0 | 4.5 ± 0.8 | 4.6 ± 0.9 | 4.7 ± 0.9 | 4.7 ± 0.9 |
| Dbase-adjusted FMD | 8.4 ± 2.0 | 7.7 ± 1.9 | 7.5 ± 2.1 | 7.3 ± 2.7 | 8.3 ± 2.0 | 9.2 ± 4.1 | 9.5 ± 2.6 | 10.4 ± 2.7 | 7.5 ± 1.9 | 7.1 ± 1.7 | 8.8 ± 2.1 | 9.3 ± 1.8 |
| Blood flow, ml/min | 144 ± 109 | 133 ± 104 | 139 ± 113 | 128 ± 98 | 127 ± 86 | 186 ± 122* | 128 ± 84 | 100 ± 53 | 116 ± 64 | 153 ± 95* | 129 ± 55 | 94 ± 74 |
| Systolic BP, mmHg | 116 ± 9 | 114 ± 11 | 104 ± 34 | 105 ± 34 | 117 ± 10 | 109 ± 9* | 101 ± 33* | 101 ± 33* | 113 ± 8 | 111 ± 6 | 99 ± 32* | 100 ± 32* |
| Diastolic BP, mmHg | 74 ± 9 | 74 ± 8 | 68 ± 22 | 68 ± 22 | 77 ± 6 | 74 ± 6 | 68 ± 22 | 68 ± 22 | 74 ± 6 | 71 ± 7 | 66 ± 22 | 67 ± 22 |
Values are mean ± SD.
Immed-ex, immediately after exercise/control; FMD, flow-mediated dilation; Dbase-adjusted FMD, allometric scaled flow-mediated dilation to diameter; BP, blood pressure.
P < 0.05 vs. CTL.
P < 0.05 vs. C-INT.
There were significant Condition × Time (P < 0.01) and Condition × Group interactions (P = 0.04) for the hyperemia-induced shear rate area under the curve (SRAUC). SRAUC did not change in the CTL condition and was not different preexercise between groups or visits. Post hoc analyses indicate significantly higher SRAUC immediately and 1 h after C-INT and immediately after R-INT compared with CTL in UN-NG and TR-NG participants (Fig. 2, all P < 0.05) but no significant changes in SRAUC were seen comparing CTL, C-INT, or R-INT at any time point in T2D participants (Fig. 2).
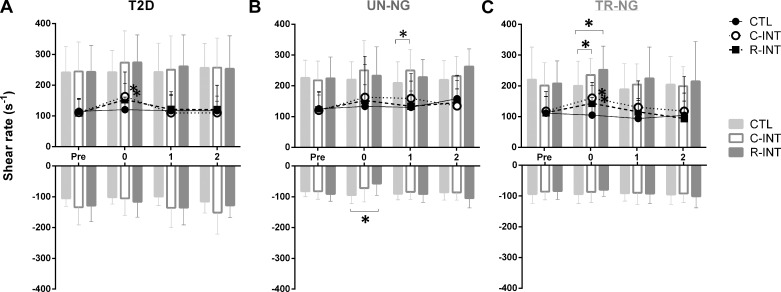
Blood Flow and Shear Rate
There were Condition × Time interactions (Table 3, P < 0.05) for baseline blood flow and baseline shear rate (Fig. 3, P < 0.05). Post hoc analyses indicate in T2D and TR-NG participants baseline shear rate was significantly higher immediately after C-INT (P < 0.05) and R-INT (P < 0.05), compared with CTL. There was a significant Condition × Time interaction (P = 0.05) for antegrade shear rate. Post hoc analyses indicate antegrade shear rate was higher in UN-NG 1 h after C-INT compared with CTL (P = 0.047). In TR-NG participants antegrade shear rate was higher immediately after R-INT (P < 0.05) and C-INT (P = 0.02), compared with CTL. There was a significant Condition × Time × Group (P = 0.048) interaction for retrograde shear rate. Post hoc analyses indicated a significantly lower retrograde flow after R-INT (P = 0.05) compared with CTL in UN-NG participants.
Fig. 3.
Baseline mean (lines), antegrade, and retrograde shear rate (s−1; bars) before, immediately, 1, and 2 h after control (CTL), C-INT, and R-INT for T2D (A), UN-NG (B), and TR-NG (C) participants. *P < 0.05, compared with CTL.
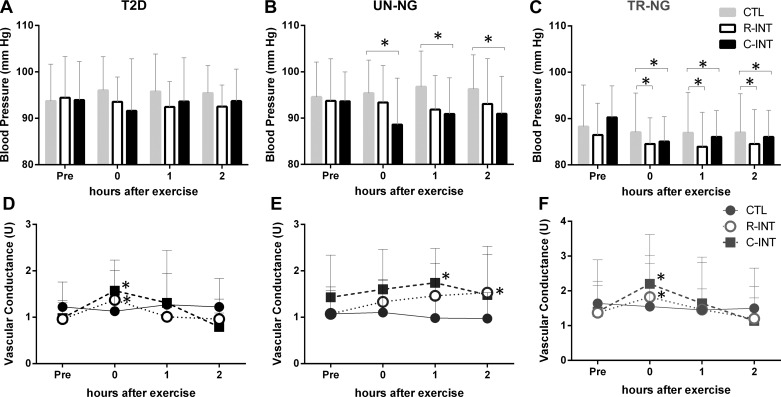
Blood Pressure and Vascular Conductance
There was a significant Condition × Time interaction (Fig. 4, P < 0.01) for mean arterial blood pressure (MAP).
Fig. 4.
Mean arterial blood pressure (MAP) and vascular conductance before, immediately, 1, and 2 h after CTL, C-INT, and R-INT in T2D (A and D), age-matched UN-NG (B and E), and TR-NG (C and F) participants. *P < 0.05, compared with CTL.
T2D.
Post hoc and inferential analyses indicated that, in T2D participants, MAP after R-INT was unchanged immediately (CI: −5.6 to 0.57 mmHg) and lower at 1 h (CI: −6.2 to −0.51 mmHg) and 2 h (CI: −5.8 to −0.03 mmHg) following exercise compared with CTL; the probability that these effects were most likely beneficial/negligible/harmful was 64/36/0.4, 84/16/0, and 75/25/0%, respectively. After C-INT, MAP in T2D was unchanged immediately (CI: −5.7 to 0.5 mmHg), 1 h (−5.0 to 0.7 mmHg), and 2 h (CI: −3.9 to 0.5 mmHg) following compared with CTL; probability of beneficial/negligible/harmful was 66/36/0, 55/45/0, and 39/61/0% respectively.
UN-NG.
MAP after R-INT in UN-NG was unchanged immediately (CI: −6.7 to 3.7 mmHg), lower at 1 h (CI: −10 to 0.2 mmHg), and unchanged 2 h (CI: −8.5 to 2.3 mmHg) following compared with CTL; probability of beneficial/negligible/harmful was 42/50/8, 89/11/0, and 70/30/3%, respectively. After C-INT exercise compared with CTL MAP in UN-NG was lower immediately (CI: −12 to −1 mmHg), 1 h (CI: −9.9 to −1.9 mmHg), and 2 h (CI: −9.4 to −1.4 mmHg) following; probability of beneficial/negligible/harmful was 95/5/0, 97/3/0, and 96/4/0%, respectively.
TR-NG.
MAP after R-INT in TR-NG was unchanged immediately (CI: −11 to 6.5 mmHg), 1 h (CI: −11 to 5.5 mmHg), and 2 h (CI: −13 to 8.3 mmHg) following compared with CTL; probability of beneficial/negligible/harmful was 54/31/15, 56/32/12, and 51/29/20%, respectively. After C-INT compared with CTL, MAP in TR-NG was unchanged immediately (CI: −3.7 to 0.3 mmHg), 1 h (CI: −4.1 to 0.7 mmHg), and 2 h (CI: −1.7 to 0.2 mmHg) following; probability of beneficial/negligible/harmful was 38/63/0, 40/60/0, and 1/99/0%, respectively.
There were significant Condition × Time interactions for both SBP (P < 0.01) and DBP (P = 0.01; Table 3). There was a significant Condition × Time interaction (Fig. 4, P = 0.05) for vascular conductance (VC). Post hoc analyses indicate in T2D and TR-NG participants VC was higher immediately after R-INT and C-INT (all P < 0.03) compared with CTL. In UN-NG participants VC was higher 1 h (P = 0.03) and 2 h (P = 0.04) after C-INT compared with CTL.
DISCUSSION
The main novel finding of this study is that resistance interval exercise (R-INT) acutely improves brachial artery endothelial function in age-matched T2D, UN-NG, and TR-NG participants. In T2D participants, %FMD was 4, 2, and 2% higher, respectively, immediately, 1 and 2 h after R-INT compared with CTL. In UN-NG and TR-NG participants, %FMD was not changed immediately after but was 2–4% higher at 1 and/or 2 h after R-INT exercise. %FMD was higher 2 h after C-INT in TR-NG participants and 1 h after C-INT in T2D, compared with CTL. The exercise-induced increases in blood flow and shear stress were similar following R-INT and C-INT, suggesting that these parameters did not fully explain the differential improvements in endothelial function. In contrast to previous research on continuous high-intensity exercise (1, 11, 25), we found no evidence of a transient period of FMD impairment following INT. These findings are important given the increasing popularity of interval exercise in clinical and nonclinical populations. Our data indicate a potential therapeutic effect of leg resistance exercise performed as INT for improving endothelial function, particularly in people with T2D. These findings warrant the examination of the long-term impact of R-INT on vascular function.
Effect of Acute Resistance INT on FMD
When compared with a time-matched seated control condition, R-INT led to higher %FMD at all time points after exercise in T2D and 1 and 2 h following R-INT in UN-NG and TR-NG participants. To the best of our knowledge this is the first study to show improved endothelial function after an acute bout of resistance type exercise. The favorable effect of R-INT for T2D and UN-NG participants may be attributed to the pattern of shear stress during resistance-based leg exercise. Indeed, it is known shear rate patterns during exercise modulate changes in endothelial function after exercise (40). Unfortunately due to technical limitations of obtaining quality images using vascular ultrasound we were not able to measure blood flow and shear rate during exercise. However, diastolic and mean arterial blood pressures were higher during R-INT compared with C-INT, suggesting the potential for greater hemodynamic-mediated shear stress during R-INT. Previous work has demonstrated that changes in endothelium-dependent dilation depend on combined increases in blood pressure and heart rate, not heart rate alone (18). However, whether there is an upper threshold for beneficial increases in pulse pressure and rate during exercise is unknown. Previous studies have shown higher exercise blood pressure with greater intensities of handgrip exercise impairs local vascular function (17, 30, 33). In the current study endothelial-dependent dilation was consistently improved after R-INT, despite significantly elevated MAP; however, the increase in MAP was ∼50% lower than Okomoto et al. (33) after handgrip exercise (peak change in MAP +17 mmHg in T2D). Discrepancies in the endothelial response to resistance exercise in our study compared with others (17, 30, 33) may also be attributed to the dynamic interval nature of the resistance exercise used in the current study, which involved a light load lifted for many repetitions (37 ± 12 reps/min) to induce fatigue and a perceived effort of “hard” (RPE of ∼5) in the last 10 s of each 1-min interval, which was followed by 1 min of recovery each time. Additionally endothelial function was measured away from the active muscle bed and it has previously been shown that upper, but not lower, limb resistance exercise increases arterial stiffness (34).
Other Potential Mechanisms Mediating FMD Responses to INT
The underlying factors modulating the changes in endothelium-dependent vasodilation after INT remain unclear. Due to the systemic nature of exercise, including interval exercise, various neurogenic, local, and hormonal stimuli may determine endothelial function. In the current study, blood flow, SRAUC (shear stimulus), baseline mean, and antegrade shear rates were elevated after both C-INT and R-INT exercise. The largest increases in blood flow and shear rate were immediately after exercise (excluding during exercise), with a time-dependent return to baseline when measured again 1 and 2 h after exercise. Shear stress is a potent stimulator of nitric oxide production and improves endothelial-dependent dilation in vivo and in vitro (40). The elevated SRAUC after C-INT and R-INT relative to CTL was lower in T2D than TR-NG and UN-NG participants (Fig. 2), but the changes in baseline blood flow, mean, antegrade, and retrograde shear rates were similar between groups and after C-INT and R-INT (Fig. 3). Similar to previous research (28) we saw no relationship between SRAUC and FMD after exercise (r = 0.00, P = 0.95). In the current study the largest improvements in FMD were seen when the hyperemic and baseline shear rate had returned near preexercise levels (Fig. 2). Elevated blood flow, shear rate, and SRAUC provide a strong stimulus for increasing endothelial nitric oxide production, mediating vasodilation (40). It is plausible that the subsequent postocclusion hyperemia immediately after exercise may not be able to cause further vasodilation as it may already be near maximally stimulated. This may explain why in the current study most improvements in endothelial function were seen 1 and/or 2 h into recovery.
Time Course and Mediators of the FMD Response to INT
It is generally reported that vigorous activities (>80% V̇o2 peak) result in a transient depression in FMD immediately after exercise (1, 11, 25). The current study saw no significant reduction in FMD after INT when performed as cardio or resistance exercise. It is thought that the transient reduction in FMD after high-intensity exercise is due to elevated sympathetic activity, changes in arterial diameter, and/or oxidative stress (reviewed in Ref. 11). The consistent improvements seen 1 and 2 h compared with immediately after INT in the current study may be due to reduced sympathetic activity 1 and 2 h postexercise and hence an improved vasodilator response. Meaningful reductions in blood pressure were seen in UN-NG participants across the 2 h after C-INT and R-INT. In addition vascular conductance was improved immediately after exercise in all groups. The sustained hyperemia after INT in the current study is an important finding and may reflect a longer lasting stimulus for favorable artery remodeling and function (41). Importantly, this response was similar in T2D, UN-NG, and TR-NG participants.
Potential Influence of Training Status
In TR-NG participants endothelial function was improved 2 h after C-INT, and 1 and 2 h after R-INT. In contrast %FMD was only significantly improved 2 h after R-INT in UN-NG participants. This finding is in agreement with others (22, 45), who show cardio-based exercise consistently improves FMD in more active participants compared with less active participants. Improvements in %FMD after both R-INT and C-INT in highly trained participants may be due to a higher antioxidant capacity to scavenge oxidants produced during high-intensity exercise, thereby increasing nitric oxide bioavailability (24). It is also important to note that the highly trained TR-NG participants in the current study performed a greater volume of exercise (higher absolute intensity but same relative intensity), for example, 85% of Wpeak for TR-NG participants was +119 W greater than T2D and +94 W greater than UN-NG participants. Although we cannot rule out any influence of higher total work, previous studies have shown the acute endothelial response does not appear to be mediated by total energy use (10, 22). Indeed Currie et al. (10) showed that %FMD was improved similarly after continuous and INT exercise, despite ∼50% lower total work for INT exercise. It is inherently difficult to match the work between groups and between resistance and cardio-based exercise. Matching the muscles used and the time and pattern of exercise was deemed more important and appropriate for this study.
Study Limitations
A consideration in the current study is that we did not measure endothelial-independent dilation (vascular smooth muscle function). However, previous studies, including two after INT exercise, show there is no change in endothelial-independent dilation following an acute bout of exercise (10, 25, 30, 40, 43). The current study design precluded endothelial-independent dilation measures to avoid potential confounding factors of repeated maximal stimulations with nitroglycerin and interactions with exercise over time.
The groups in this study are matched by age only; therefore, we cannot rule out any influence of body mass, medications, or long-term diet on blood flow and endothelial responses to exercise. Age was considered by the authors to be the most important and pragmatic variable to match while examining whether the presence of T2D and/or fitness (training status) influenced the changes in endothelial function after two modes of acute interval exercise. It would be quite difficult to find obese adults with no metabolic or cardiovascular risk factors that engaged in 2.5–5 h and >7 h of exercise training per week so groups were matched on age only.
Increases in blood flow and shear rate during exercise can cause vasodilation through local regulatory mechanisms that may influence baseline diameter, which may confound the %FMD calculation (36). To adjust for changes in baseline diameter, allometric scaling was used according to current recommendations (2). The same significant relationship as %FMD was seen for FMD corrected for diameter in T2D after R-INT. However, for TR-NG participants the changes in FMD after R-INT and C-INT when corrected for diameter were no longer significant, despite similar trends as %FMD.
It is important to note that the T2D participants had completed a brief familiarization period before these acute investigations, as they were participating in a longer-term study (NCT02251301). This involved six sessions of INT; four 1-min intervals eliciting an RPE of ∼5 were performed in the first three sessions; thereafter, the number increased by one interval each session until they reached six intervals. This was deemed necessary to ensure the T2D participants could complete seven 1-min interval, were accustomed to this type of vigorous exercise, and did not experience any abnormal heart rate or blood pressure responses to INT. Endothelial function measured before and after the 2-wk habituation period was unchanged (+0.5 ± 2.4%, P = 0.50, data not shown), however, the endothelial responses seen in the current study may not generalize to inactive T2D participants or those completely naïve to INT.
Conclusions
In conclusion, this study shows that resistance-based interval exercise is a time-efficient and effective exercise method to acutely improve endothelial function in T2D and age-matched UN-NG and TR-NG participants. This is the first study to investigate the acute effect of this novel form of INT and demonstrates its potential utility in older adults with and without T2D. Although the mechanisms underlying the changes in endothelial function with cardio- and resistance-based INT are unclear, the pattern of high- and low-intensity exercise stimulates an increase in blood flow and shear rate postexercise and did not cause a transient decrease in endothelial function as found previously for continuous vigorous exercise. The chronic effects of repeated resistance-based vs. cardio-based INT warrant investigation to elucidate whether these acute responses transpire to long-term vascular adaptations in these groups.
GRANTS
Funding for this study was provided by a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant RGPIN 435807-13 (to J. P. Little). J. P. Little is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Salary Award (FRN, MSH- 141980).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.E.F. and J.P.L. conception and design of research; M.E.F. and C.D. performed experiments; M.E.F., K.P., and F.H. analyzed data; M.E.F. and J.P.L. interpreted results of experiments; M.E.F. prepared figures; M.E.F. drafted manuscript; M.E.F. and J.P.L. edited and revised manuscript; M.E.F., C.D., K.P., F.H., and J.P.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jacqueline Gabelhouse and Jordelle Dupre from COACH cardiology for assistance with the 12-lead exercise stress tests. Importantly, we also thank all the participants for giving the time to participate in this research.
REFERENCES
- 1.Atkinson CL, Lewis N, Carter HH, Thijssen DH, Ainslie PN, Green DJ. Impact of sympathetic nervous system activity on post-exercise flow-mediated dilatation in humans. J Physiol 593: 5145–5156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson G, Batterham AM. The percentage flow-mediated dilation index: a large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc Med 18: 354–365, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Int J Sports Physiol Perform 1: 50–57, 2006. [PubMed] [Google Scholar]
- 4.Bird SR, Hawley JA. Exercise and type 2 diabetes: new prescription for an old problem. Maturitas 72: 311–316, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Borg GA. Physical Performance and Perceived Exertion. Lund, Sweden: Gleerup, 1962. [Google Scholar]
- 7.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA 294: 2981–2988, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 155: 701–709, 1995. [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Currie KD, McKelvie RS, MacDonald MJ. Flow-mediated dilation is acutely improved after high-intensity interval exercise. Med Sci Sports Exerc 44: 2057–2064, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol 115: 1589–1598, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction testing and clinical relevance. Circulation 115: 1285–1295, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Francois ME, Baldi JC, Manning PJ, Lucas SJ, Hawley JA, Williams MJ, Cotter JD. ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 57: 1437–1445, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Francois ME, Little JP. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectrum 28: 39–44, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillen J, Little J, Punthakee Z, Tarnopolsky M, Riddell M, Gibala M. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metab 14: 575–577, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Gillen JB, Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl Physiol Nutr Metab 39: 409–412, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Gonzales JU, Thompson BC, Thistlethwaite JR, Scheuermann BW. Association between exercise hemodynamics and changes in local vascular function following acute exercise. Appl Physiol Nutr Metab 36: 137–144, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Green D, Cheetham C, Henderson C, Weerasooriya R, O'Driscoll G. Effect of cardiac pacing on forearm vascular responses and nitric oxide function. Am J Physiol Heart Circ Physiol 283: H1354–H1360, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Hadi HA, Al Suwaidi J. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manage 3: 853, 2007. [PMC free article] [PubMed] [Google Scholar]
- 21.Hallmark R, Patrie JT, Liu Z, Gaesser GA, Barrett EJ, Weltman A. The effect of exercise intensity on endothelial function in physically inactive lean and obese adults. PLoS One 9: e85450, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity 16: 578–584, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Ji LL. Antioxidants and oxidative stress in exercise. Exp Biol Med (Maywood) 222: 283–292, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BD, Padilla J, Wallace JP. The exercise dose affects oxidative stress and brachial artery flow-mediated dilation in trained men. Eur J Appl Physiol 112: 33–42, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Jones H, Green DJ, George K, Atkinson G. Intermittent exercise abolishes the diurnal variation in endothelial-dependent flow-mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol 298: R427–R432, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551–5558, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llewellyn T, Chaffin M, Berg K, Meendering J. The relationship between shear rate and flow-mediated dilation is altered by acute exercise. Acta Physiol (Oxf) 205: 394–402, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol 38: 860–866, 2001. [DOI] [PubMed] [Google Scholar]
- 30.McGowan CL, Levy AS, Millar PJ, Guzman JC, Morillo CA, McCartney N, MacDonald MJ. Acute vascular responses to isometric handgrip exercise and effects of training in persons medicated for hypertension. Am J Physiol Heart Circ Physiol 291: H1797–H1802, 2006. [DOI] [PubMed] [Google Scholar]
- 31.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. New York: Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance a randomized intervention study. Circulation 110: 2858–2863, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto T, Masuhara M, Ikuta K. Relationship between plasma endothelin-1 concentration and cardiovascular responses during high-intensity eccentric and concentric exercise. Clinl Physiol Function Imaging 28: 43–48, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto T, Masuhara M, Ikuta K. Upper but not lower limb resistance training increases arterial stiffness in humans. Eur J Appl Physiol 107: 127–134, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Olson TP, Dengel DR, Leon AS, Schmitz KH. Moderate resistance training and vascular health in overweight women. Med Sci Sports Exerc 38: 1558–1564, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Padilla J, Harris RA, Wallace JP. Can the measurement of brachial artery flow-mediated dilation be applied to the acute exercise model? Cardiovasc Ultrasound 5: 1, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schjerve IE, Tyldum GA, Tjønna AE, Stølen T, Loennechen JP, Hansen HE, Haram PM, Heinrich G, Bye A, Najjar SM. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci 115: 283–293, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Thijssen D, Dawson EA, Black MA, Hopman M, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc 41: 1072–1079, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay MS, Warburton DE, Janssen I, Paterson DH, Latimer AE, Rhodes RE, Kho ME, Hicks A, LeBlanc AG, Zehr L. New Canadian physical activity guidelines. Appl Physiol Nutr Metab 36: 36–46, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Tyldum GA, Schjerve IE, Tjønna AE, Kirkeby-Garstad I, Stølen TO, Richardson RS, Wisløff U. Endothelial dysfunction induced by postprandial lipemia: complete protection afforded by high-intensity aerobic interval exercise. J Am Coll Cardiol 53: 200–206, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vona M, Codeluppi G, Iannino T, Ferrari E, Bogousslavsky J, Von Segesser L. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation 119: 1601–1608, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Walther G, Nottin S, Karpoff L, Pérez-Martin A, Dauzat M, Obert P. Flow-mediated dilation and exercise-induced hyperaemia in highly trained athletes: comparison of the upper and lower limb vasculature. Acta Physiol (Oxf) 193: 139–150, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48: 1227–1234, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients a randomized study. Circulation 115: 3086–3094, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Woodman R, Playford D, Watts G, Cheetham C, Reed C, Taylor R, Puddey I, Beilin L, Burke V, Mori T. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]