Fig. 3.
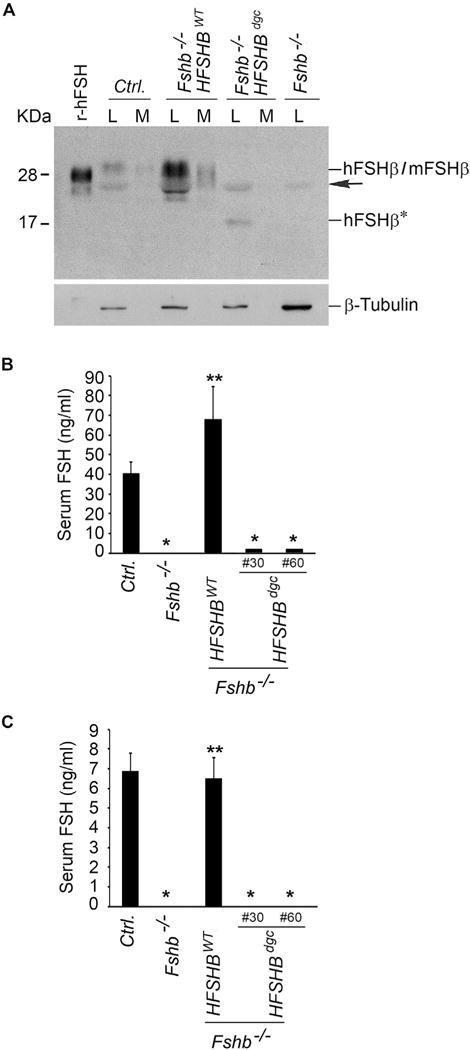
Western blot analysis (A) of proteins in lysates and medium fractions derived from whole pituitary organ cultures separated under non-reducing conditions and probed with 4B FSH monoclonal antibody, indicated that interspecies hybrid heterodimer containing the non-glycosylated hFSHβ mutant subunit (in Fshb−/− HSFHBdgc mouse pituitary), was barely secreted into medium compared to the hFSHβ WT subunit-containing interspecies hybrid heterodimer (in Fshb−/− HSFHBWT mouse pituitary) or mouse FSH heterodimer from control normal mouse pituitary. The black arrow indicates a non-specific band. Fshb−/− mouse pituitary lysate and r-hFSH served as negative and positive controls, respectively. Expression of β-tubulin was used as an internal control and is shown in the blot at the bottom of panel A. L: lysate; M: medium; hFSHβ*: non-glycosylated hFSHβAsn7Δ Asn24Δ double site mutant subunit. RIAs on serum samples from adult male (B) and female (C) mice revealed very low levels of non-glycosylated hFSHβAsn7Δ Asn24Δ double site mutant subunit-containing FSH dimer in two lines (Line #30 and Line #60) of Fshb−/− HSFHBdgc mice compared to that in normal control (Ctrl.) or Fshb−/− HSFHBWT mice expressing hFSHβ WT subunit. In panels B and C: * P < 0.01 compared to Ctrl. or Fshb−/− HSFHBWT groups; ** P < 0.01 compared to Fshb−/− group.
