Figure 9. Hypothetical model of p17 secretion from Gag-expressing cells.
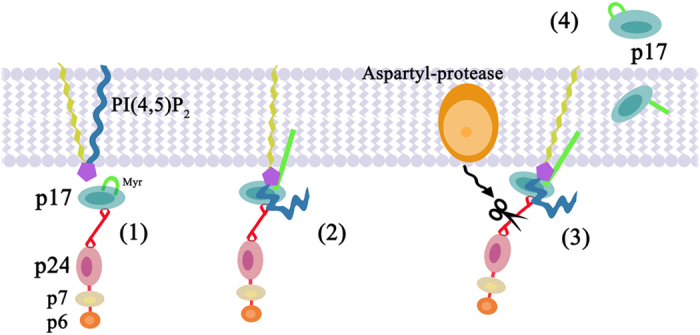
The cytosolic Pr55Gag, a polyprotein composed of the matrix protein p17 − whose NH2-terminal myristic acid moiety in its sequestered conformation is in light green −, the capsid protein p24, the nucleocapsid protein p7, and the p6 domains (1), is recruited to the cellular membrane by PI(4,5)P2, according to the model proposed by Saad et al.26 (2). PI(4,5)P2 association with the p17 highly basic domain induces a conformational change enabling the membrane-embedded aspartyl-protease to cleave p17 from the polyprotein Pr55Gag (3). As a Pr55Gag-free protein, p17 then moves to the extracellular space through an unconventional secretion mechanism (4).
